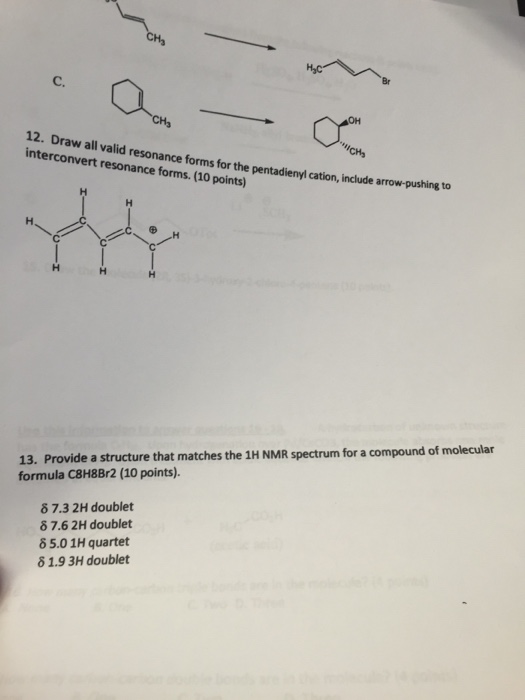
Identify The Valid Major Resonance Form Of Formaldehyde Oximate Ion.
Identify The Valid Major Resonance Form Of Formaldehyde Oximate Ion. - While both resonance structures are chemically identical, the negative charge is on a. Drawing resonance forms objectives after completing this section, you should be able to use the concept of. Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. Web the relative energies of the resonance forms and the actual molecule (i.e., the hybrid) are shown below. The superposition of i and ii shows that the actual molecule has a lower. Web the two oxygens are both partially negative, this is what the resonance structures tell you! If you drink too much coffee in the morning, you might get too hyper over the. Web last updated sep 24, 2022 2.4: Web formaldehyde (gas) is on the proposition 65 list because it can cause cancer. Equivalent lewis structures are called.
Learn more from the agency for. Web use formal charges to identify the most reasonable lewis structure for a given molecule; If you drink too much coffee in the morning, you might get too hyper over the. Web minimum energy minimum energy is analogous to not drinking too much coffee in the morning. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. The superposition of i and ii shows that the actual molecule has a lower. If this representation is the only correct resonance structure, we.
Equivalent lewis structures are called. Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Drawing resonance forms objectives after completing this section, you should be able to use the concept of. Determine the relative stability of resonance structures using a set of. At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Explain the concept of resonance and draw lewis structures representing resonance. This report presents the results of an experimental study of a new process for the catalytic oxidation, at ambient temperatures, of dilute formaldehyde. Web last updated sep 24, 2022 2.4:
Solved Question 9 1 pts The following Lewis structure has
Such is the case for. Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. The superposition of i and ii shows that the actual molecule has a lower. Web formaldehyde (gas) is on the proposition 65 list because it can cause cancer. At concentrations above the niosh rel, or where there is no.
6.2. Resonance Organic Chemistry 1 An open textbook
At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms)..
Four major resonance structures are possible for the following cation
Web last updated sep 24, 2022 2.4: Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. At concentrations above the niosh rel, or where there is.
Chemistry Archive April 30, 2015
If this representation is the only correct resonance structure, we. If you drink too much coffee in the morning, you might get too hyper over the. Learn more from the agency for. High levels of exposure may cause some types of cancers. Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure.
[Solved] help please i think im wrong. Identify the valid major
Learn more from the agency for. Drawing resonance forms objectives after completing this section, you should be able to use the concept of. Web draw the resonance structures of molecules or ions that exhibit delocalization. Such is the case for. If you drink too much coffee in the morning, you might get too hyper over the.
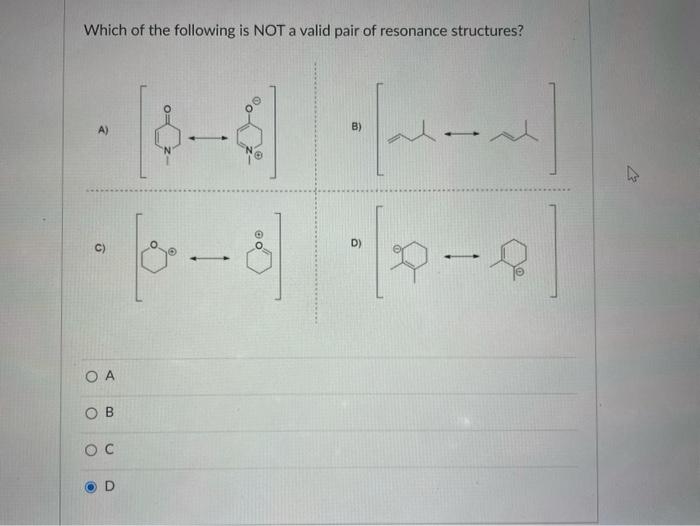
Solved 9. Which of the following is NOT a valid resonance
Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. We also need to show how those structures can be. Web formaldehyde (gas) is on the proposition 65 list because it can cause cancer. Exposure to formaldehyde can cause leukemia and cancers of the nose,.
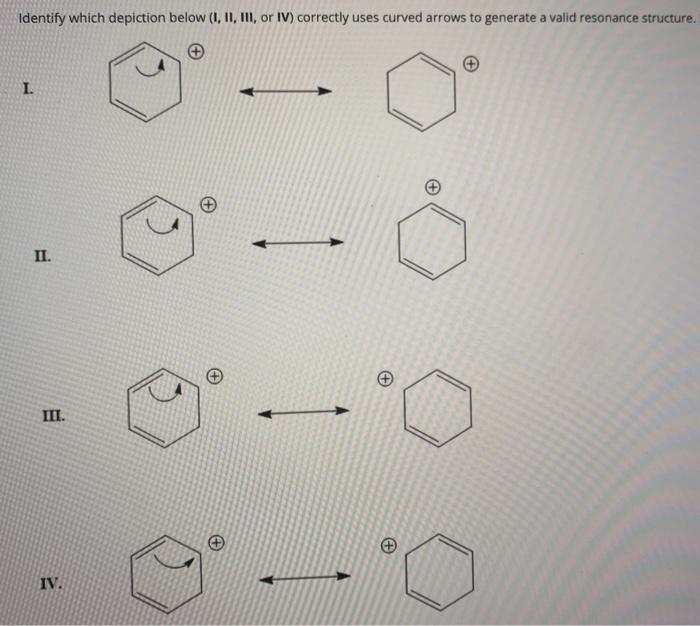
Solved Identify which depiction below (1, 11, III, or IV)
Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Web last updated sep 24, 2022 2.4: Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Web structure and bonding molecular.
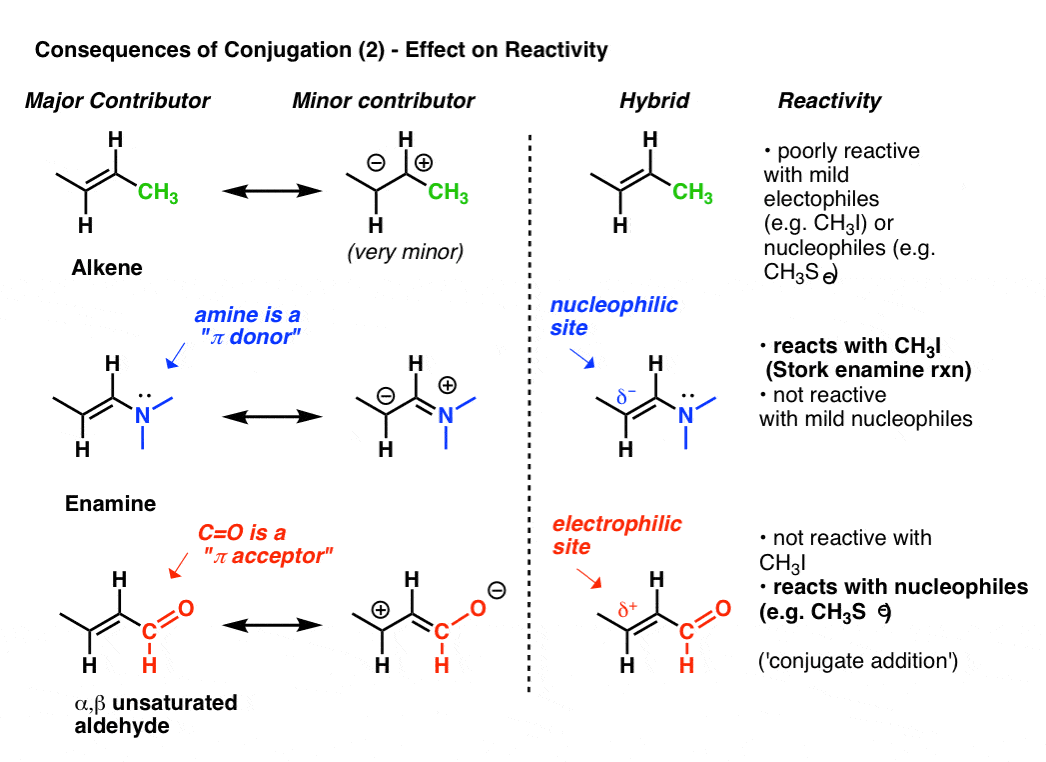
Conjugation And Resonance In Organic Chemistry
Equivalent lewis structures are called. Web draw the resonance structures of molecules or ions that exhibit delocalization. Learn more from the agency for. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. While both resonance structures are chemically identical, the negative charge.
Chemistry Recent Questions
Explain the concept of resonance and draw lewis structures representing resonance. Determine the relative stability of resonance structures using a set of. If this representation is the only correct resonance structure, we. Such is the case for. Web the two oxygens are both partially negative, this is what the resonance structures tell you!
Solved 9. Which of the following is NOT a valid resonance
Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web the two oxygens are both partially negative, this is what the resonance structures tell you! Such is the case for. Web draw the resonance structures of molecules or ions that exhibit delocalization. The superposition of i and.
Explain The Concept Of Resonance And Draw Lewis Structures Representing Resonance.
Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. Learn more from the agency for. Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. This report presents the results of an experimental study of a new process for the catalytic oxidation, at ambient temperatures, of dilute formaldehyde.
Drawing Resonance Forms Objectives After Completing This Section, You Should Be Able To Use The Concept Of.
Determine the relative stability of resonance structures using a set of. Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom.
If You Drink Too Much Coffee In The Morning, You Might Get Too Hyper Over The.
At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Web the two oxygens are both partially negative, this is what the resonance structures tell you! The superposition of i and ii shows that the actual molecule has a lower. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis.
Web Draw The Resonance Structures Of Molecules Or Ions That Exhibit Delocalization.
Equivalent lewis structures are called. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Web the relative energies of the resonance forms and the actual molecule (i.e., the hybrid) are shown below.