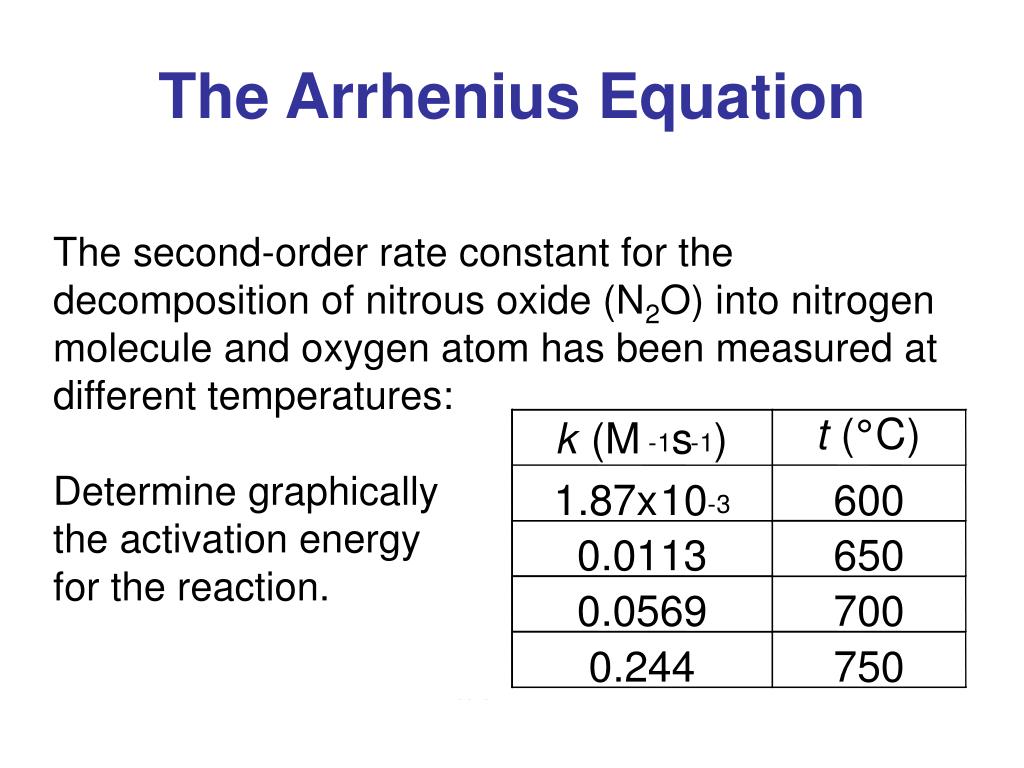
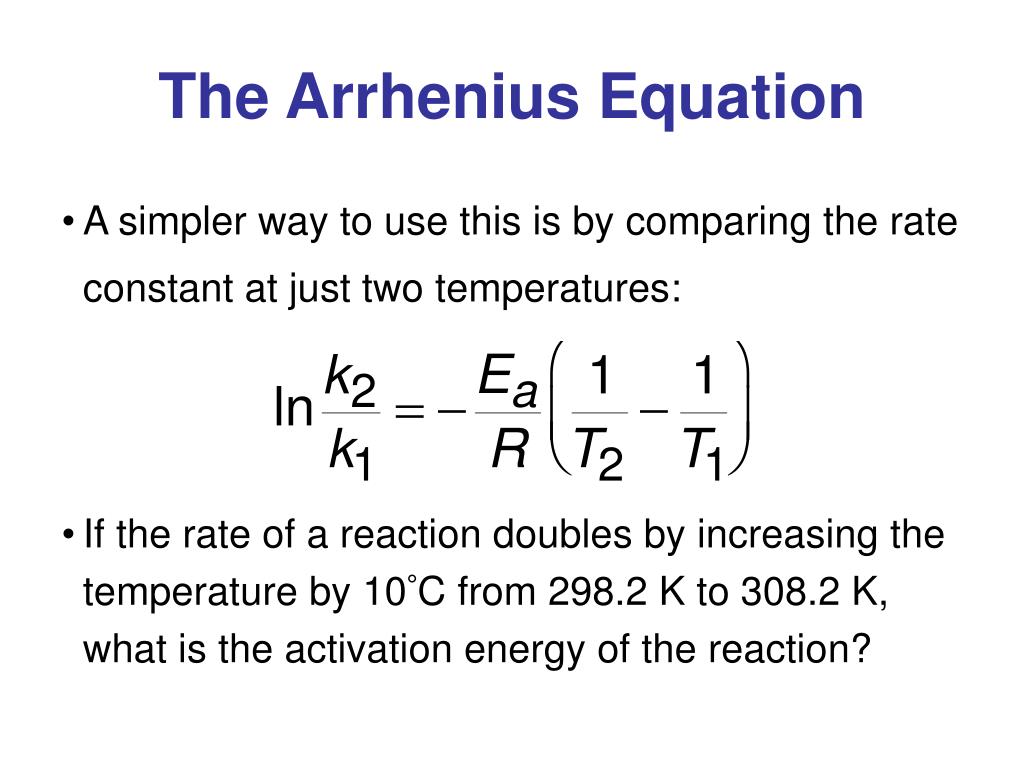
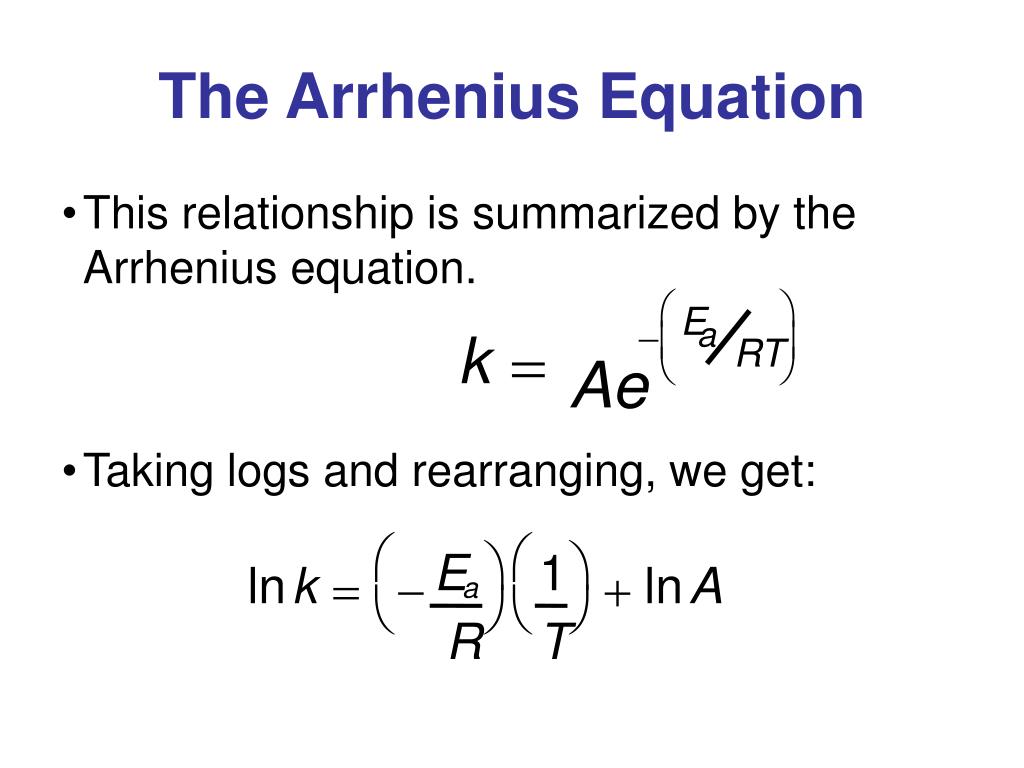
Arrhenius Equation In Log Form
Arrhenius Equation In Log Form - Web arrhenius equation describes the relation between the rate of reaction and temperature for many physical and chemical reactions. Ln k 1 = ln (a). Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. Web the arrhenius equation can be expressed in a more applicable form by taking the natural logarithm of both sides which gives a form of a linear equation: A common form of the equation is (6.10) k e ( e. Web answer (1 of 3): Logarithmic form of arrhenius equation or method to find activation energy. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate. In other words, you can use \log_{10} for the arrhenius equation, just be. Take the natural log ln(k ) ln a e e a.
Web answer (1 of 3): R.t from example 21.8 in kask and rawn. Logarithmic form of arrhenius equation or method to find activation energy. A common form of the equation is (6.10) k e ( e. When we plot the log of the rate constant, ln k on the. Web the above equation is the arrhenius equation. This relates to the general equation of a line, y = mx +c, and can be plotted on. Ln k 1 = ln (a). Web arrhenius equation describes the relation between the rate of reaction and temperature for many physical and chemical reactions. Take the natural log ln(k ) ln a e e a.
When we plot the log of the rate constant, ln k on the. Web arrhenius equation the arrhenius equation k a e e a. This relates to the general equation of a line, y = mx +c, and can be plotted on. In other words, you can use \log_{10} for the arrhenius equation, just be. Web the logarithmic form of the arrhenius equation for a chemical reaction at two different temperatures t1 and t2, with corresponding rate constants k1 and k2, is: A common form of the equation is (6.10) k e ( e. Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. Logarithmic form of arrhenius equation or method to find activation energy. Web answer (1 of 3): This means a plot of ln k.
Class12. Chemical / log form of Arrhenius equation (derivation
Is it because writing the extra factor of 2.303 every time becomes inconvenient? When we plot the log of the rate constant, ln k on the. Web the above equation is the arrhenius equation. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate. Ln.
PPT The Arrhenius Equation PowerPoint Presentation, free download
R.t from example 21.8 in kask and rawn. Web arrhenius equation the arrhenius equation k a e e a. Web the logarithmic form of the arrhenius equation for a chemical reaction at two different temperatures t1 and t2, with corresponding rate constants k1 and k2, is: When we plot the log of the rate constant, ln k on the. Web.
Spice of Lyfe Arrhenius Equation Chemical
R.t from example 21.8 in kask and rawn. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate. Web answer (1 of 3): This means a plot of ln k. When we plot the log of the rate constant, ln k on the.
PPT The Arrhenius Equation PowerPoint Presentation, free download
A common form of the equation is (6.10) k e ( e. Web arrhenius equation the arrhenius equation k a e e a. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate. Web the above equation is the arrhenius equation. In other words, you.
In the Arrhenius equation, `k = A exp^(Ea//RT)`, A may be termed as
Ln k 1 = ln (a). This relates to the general equation of a line, y = mx +c, and can be plotted on. Web arrhenius equation the arrhenius equation k a e e a. Web the arrhenius equation can be expressed in a more applicable form by taking the natural logarithm of both sides which gives a form of.
arrhenius equation Yahoo Image Search Results Gas constant
R.t from example 21.8 in kask and rawn. Is it because writing the extra factor of 2.303 every time becomes inconvenient? A common form of the equation is (6.10) k e ( e. This relates to the general equation of a line, y = mx +c, and can be plotted on. Web arrhenius equation the arrhenius equation k a e.
PPT The Arrhenius Equation PowerPoint Presentation, free download
When we plot the log of the rate constant, ln k on the. Web the logarithmic form of the arrhenius equation for a chemical reaction at two different temperatures t1 and t2, with corresponding rate constants k1 and k2, is: Logarithmic form of arrhenius equation or method to find activation energy. Web arrhenius equation the arrhenius equation k a e.
16.2 The Arrhenius equation (HL) YouTube
Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. R.t from example 21.8 in kask and rawn. Ln k 1 = ln (a). In other words, you can use \log_{10} for the arrhenius equation, just be. Web.
PPT The Arrhenius Equation PowerPoint Presentation, free download
Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. In other words, you can use \log_{10} for the arrhenius equation, just be. Is it because writing the extra factor of 2.303 every time becomes inconvenient? A common.
Arrhenius Equation YouTube
Web the logarithmic form of the arrhenius equation for a chemical reaction at two different temperatures t1 and t2, with corresponding rate constants k1 and k2, is: Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. Ln.
When We Plot The Log Of The Rate Constant, Ln K On The.
Ln k 1 = ln (a). A common form of the equation is (6.10) k e ( e. Web the logarithmic form of the arrhenius equation for a chemical reaction at two different temperatures t1 and t2, with corresponding rate constants k1 and k2, is: Web arrhenius equation describes the relation between the rate of reaction and temperature for many physical and chemical reactions.
R.t From Example 21.8 In Kask And Rawn.
Web the above equation is the arrhenius equation. Take the natural log ln(k ) ln a e e a. Is it because writing the extra factor of 2.303 every time becomes inconvenient? This relates to the general equation of a line, y = mx +c, and can be plotted on.
Web The Arrhenius Activation Energy For Two Temperature Calculator Uses The Arrhenius Equation To Compute Activation Energy Based On Two Temperatures And Two Reaction Rate.
Web arrhenius equation the arrhenius equation k a e e a. Web solution if the reaction rate is twice as fast, fun facts/ additional information a graph of data used to generate the arrhenius equation is called an arrhenius plot, where the x. Logarithmic form of arrhenius equation or method to find activation energy. In other words, you can use \log_{10} for the arrhenius equation, just be.
This Means A Plot Of Ln K.
Web answer (1 of 3): Web the arrhenius equation can be expressed in a more applicable form by taking the natural logarithm of both sides which gives a form of a linear equation: