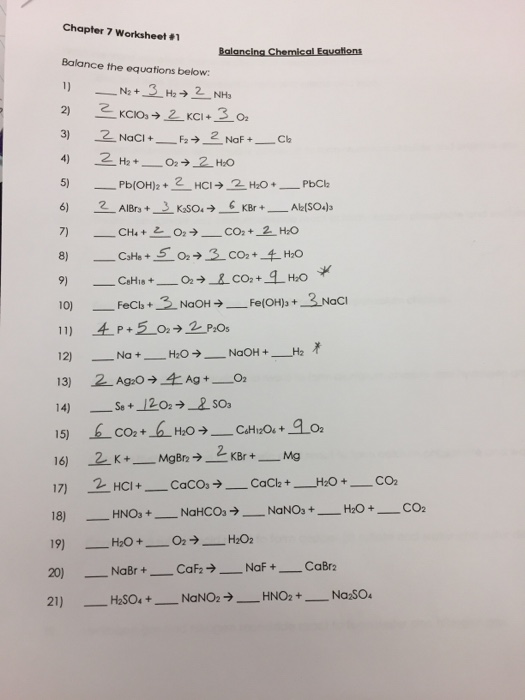Balancing Chemical Equations Chapter 7 Worksheet 1
Balancing Chemical Equations Chapter 7 Worksheet 1 - The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). 4 balancing equations worksheets with answers; Web showing 8 worksheets for balancing chemical equations. 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f2 æ ____ naf + ____ cl2 4) ____ h2 + ____ o2 æ ____ h2o 5) ____ pb(oh)2 + ____ hcl. Web balance the following equations: Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: Worksheets are balancing equations practice problems, chapter 7 work 1 balancing chemical equat. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1 1 n2 3 h2 2 nh3 2 2 kclo3 3 2.
Pcl5(s) +h2o(l) → pocl3(l) + hcl(aq) pcl 5 ( s) + h 2 o ( l) → pocl 3 ( l) + hcl ( a q) cu(s) +hno3(aq) → cu(no3)2(aq) +h2o(l) + no(g) cu ( s) + hno 3 ( a q) →. Worksheets are balancing equations practice problems, chapter 7 work 1 balancing chemical equat. Web chapter 7 balancing chemical equation. Web 2 balancing chemical equations worksheets; 1) ____ n2 + ____ h2 2 nh3 ____ 2) 2 kclo3 ____ ____ kcl + ____ o2 3) ____ 2 nacl + ____ f2 ____ naf + ____ 1 cl2 4) ____ h2 + ____ o2. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). This is a requirement the. 8 methods you can use for balancing chemical equation;. Write the expression for k_ {\mathrm {sp}} k sp for this process. Web we have 14 worksheets about chapter 7 worksheet 1 balancing chemical equations including images, pictures, photos, wallpapers, and more.
Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: Web chapter 7 balancing chemical equation. 1) ___1__ al(no 3) 3 + __1___ (nh 4) 3 po 4 → ___1__alpo 4 + _3_ nh 4 no 3. Write the expression for k_ {\mathrm {sp}} k sp for this process. Section 7.3 energy changes in reactions. 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f2 æ ____ naf + ____ cl2 4) ____ h2 + ____ o2. 5 what are different types of chemical equations? Web showing 8 worksheets for balancing chemical equations. 4 balancing equations worksheets with answers; 1) ____ n2+ ____ h2æ ____ nh3 2) ____ kclo3æ ____ kcl + ____ o2 3) ____ nacl + ____ f 2æ____ naf + ____ cl 2 4) ____ h2+ ____ o2æ ____ h2o 5) ____ pb (oh) 2+ ____ hclæ____ h2o + ____ pbcl 2 6) ____ albr3 + ____ k2so4æ____ kbr + ____ al2(so4)3 7…
30 Balancing Chemical Equations Worksheet 1 Education Template
In these page, we also have variety of worksheets available. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: We have 16 worksheets about balancing chemical.
Chapter 7 Worksheet 1 Balancing Chemical Equations 49 —
Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1) ____ n2+ ____ h2 æ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f 2æ ____ naf + ____ cl 2 4) ____ h2 + ____ o2æ ____ h2o 5) ____ pb (oh) 2 + ____ hcl æ ____ h2o.
49 Balancing Chemical Equations Worksheets [with Answers]
Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1 1 n2 3 h2 2 nh3 2 2 kclo3 3 2. Writing and balancing chemical reactions 1. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product.
Chapter 7 Worksheet 1 Balancing Chemical Equations
7 how to balance a chemical equation? Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1) ____ n 2 + 4 h 2 → 2 nh 3 2) 2 kclo 3 → 2 kcl + 3 o 2 3) 2 nacl + ____ f 2 → 2 naf + ____ cl 2 4) 2 h 2.
30 Balancing Chemical Equations Worksheet 1
The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). 8 methods you can use for balancing chemical equation;. 1) ____ n2+ ____ h2æ ____ nh3 2) ____ kclo3æ ____ kcl + ____ o2 3) ____ nacl + ____ f 2æ____ naf + ____ cl.
Worksheet More Practice Balancing Equations Balance The Following
Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Web chapter 7 balancing chemical equation. 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____.
Chemistry Balancing Equations Worksheet Answer Key / Answer Key
Pcl5(s) +h2o(l) → pocl3(l) + hcl(aq) pcl 5 ( s) + h 2 o ( l) → pocl 3 ( l) + hcl ( a q) cu(s) +hno3(aq) → cu(no3)2(aq) +h2o(l) + no(g) cu ( s) + hno 3 ( a q) →. Alejandro jimenez _____ balance the following equations: 3 why is it important to balance the chemical equations?.
Chapter 7 Worksheet 1 Balancing Chemical Equations —
Web chem 101 chapter 7 worksheet 1 balancing chemical equations name: Pcl5(s) +h2o(l) → pocl3(l) + hcl(aq) pcl 5 ( s) + h 2 o ( l) → pocl 3 ( l) + hcl ( a q) cu(s) +hno3(aq) → cu(no3)2(aq) +h2o(l) + no(g) cu ( s) + hno 3 ( a q) →. Worksheets are balancing equations practice problems,.
Balancing Chemical Equations Chapter 7 Worksheet 1
8 methods you can use for balancing chemical equation;. This is a requirement the. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 7 how to balance a chemical equation? Web chapter 7 worksheet #1 balancing chemical equations balance the equations below:
Write The Balanced Chemical Equation Describing The Dissolving Of \Mathrm {Mgco}_3 (S) Mgco3(S) In Water.
7 how to balance a chemical equation? 1) ____ n2+ ____ h2 æ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f 2æ ____ naf + ____ cl 2 4) ____ h2 + ____ o2æ ____ h2o 5) ____ pb (oh) 2 + ____ hcl æ ____ h2o + ____ pbcl 2 6) ____ albr3 + ____ k2so4 æ____ kbr + ____ al2(so4)3 7… 1) ____ n 2 + 4 h 2 → 2 nh 3 2) 2 kclo 3 → 2 kcl + 3 o 2 3) 2 nacl + ____ f 2 → 2 naf + ____ cl 2 4) 2 h 2 + ____ o 2 → 2 h 2 o 5). Web we have 14 worksheets about chapter 7 worksheet 1 balancing chemical equations including images, pictures, photos, wallpapers, and more.
8.4 Strength Of Acids And Bases.
Section 7.3 energy changes in reactions. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 5 what are different types of chemical equations? 4 balancing equations worksheets with answers;
Web 2 Balancing Chemical Equations Worksheets;
1) ____ n2+ ____ h2æ ____ nh3 2) ____ kclo3æ ____ kcl + ____ o2 3) ____ nacl + ____ f 2æ____ naf + ____ cl 2 4) ____ h2+ ____ o2æ ____ h2o 5) ____ pb (oh) 2+ ____ hclæ____ h2o + ____ pbcl 2 6) ____ albr3 + ____ k2so4æ____ kbr + ____ al2(so4)3 7… 1) _ 1 ___ n 2 + __ 3_ _ h 2 → ___ 2 _ nh 3 2) __ 2 __ kclo 3 → __ 2 __ kcl + __ 3_ _ o 2 3) __ 2_ _ nacl + __ 1 __ f 2 → __ 2 __ naf + __ 1 __ cl 2 4) __ 2 __ h 2 + __ 1. Balance the following equations and indicate the type of reaction as formation, decomposition, single. We have 16 worksheets about balancing chemical equations chapter 7 worksheet 1 including images, pictures, photos, wallpapers,.
Write The Expression For K_ {\Mathrm {Sp}} K Sp For This Process.
3 why is it important to balance the chemical equations? 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f2 æ ____ naf + ____ cl2 4) ____ h2 + ____ o2. 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2 3) ____ nacl + ____ f2 æ ____ naf + ____ cl2 4) ____ h2 + ____ o2 æ ____ h2o 5) ____ pb(oh)2 + ____ hcl. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides.


![49 Balancing Chemical Equations Worksheets [with Answers]](https://i.pinimg.com/originals/95/4f/dd/954fddc7c6a4215236c9f75fcb3dc32f.jpg)