Can Nitrogen Form 4 Bonds
Can Nitrogen Form 4 Bonds - Web can nitrogen form 4 bonds. So if it has 4 bonds it'll have a charge of +1 (b/c it has 1 more bond than its preferred #). This coordinate bond that nitrogen forms by. Web nitrogen's valenc number is 3. However, in the case of n20, n forms a triple bond with the second n (the central atom) and that second. Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four. Carbon can form four covalent bonds to create an organic molecule. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms.
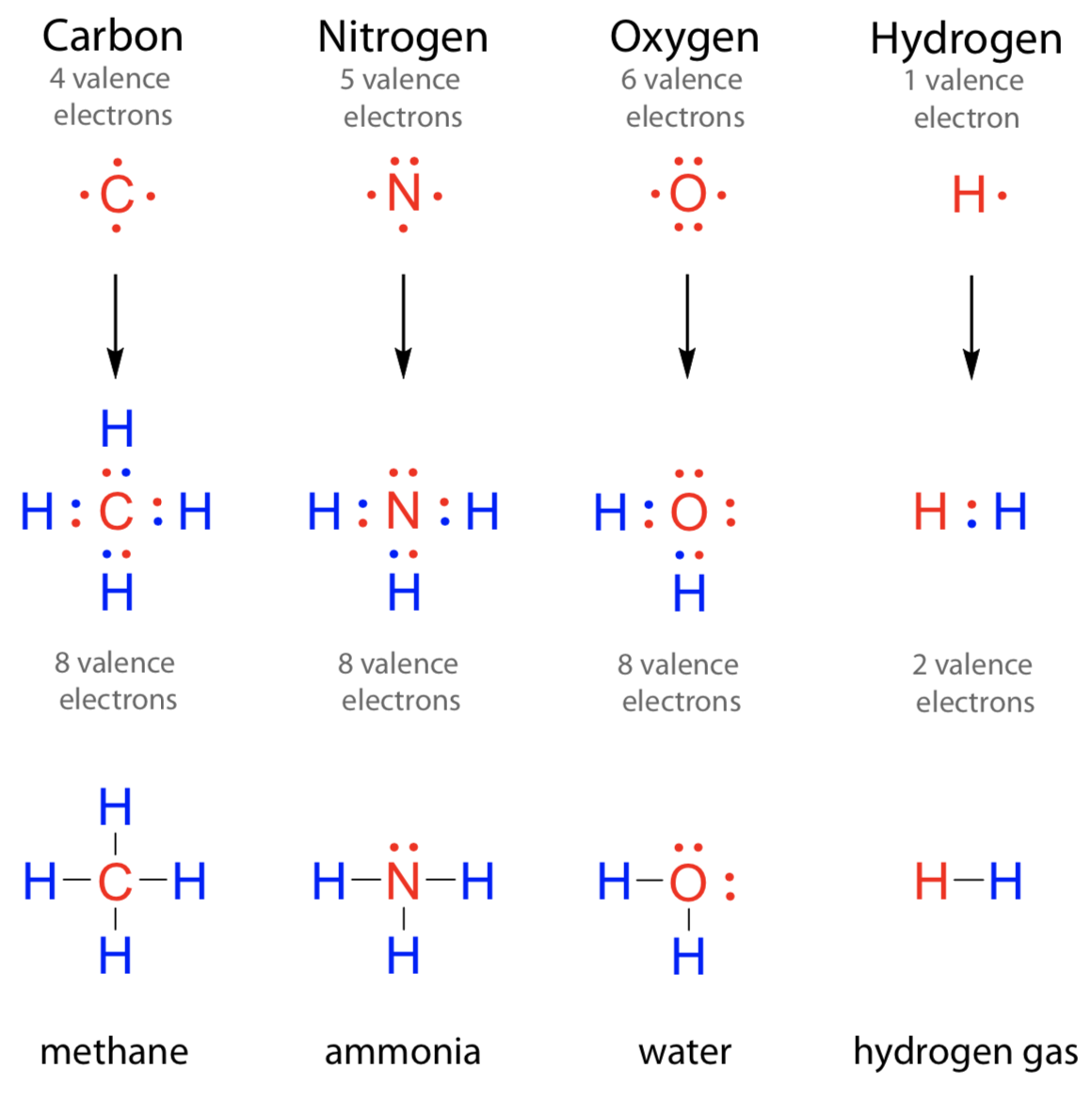
Group 5a (15) elements such as nitrogen. Web nitrogen will usually have 3 bonds, occasionally 4; Web nitrogen's valenc number is 3. This coordinate bond that nitrogen forms by. Carbon can form four covalent bonds to create an organic molecule. Web i was told that nitrogen can form only up to three bonds. Two core and five valence: It can donate this electron pair to form a coordinate bond. So if it has 4 bonds it'll have a charge of +1 (b/c it has 1 more bond than its preferred #). The simplest carbon molecule is methane (ch 4 ), depicted here.
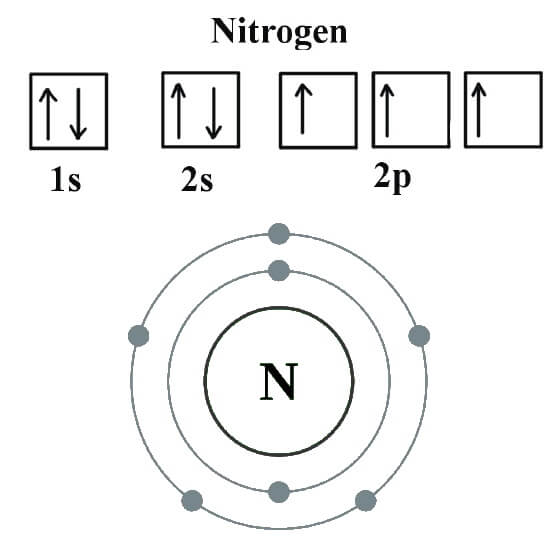
However, in the case of n20, n forms a triple bond with the second n (the central atom) and that second. This coordinate bond that nitrogen forms by. Web nitrogen's valenc number is 3. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web nitrogen atom can attain an octet configuration by sharing three electrons with another nitrogen atom, forming a triple bond (three pairs of electrons shared) diagram of. Web nitrogen has one lone pair of electrons in its 2s orbital. So if you are following the rules, you might well assume that nitrogen would be able to form five. Web nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. In diazonium ion n has 4 bonds, then one of those 4.
How Many Bonds Can Nitrogen Form Jacks Of Science
Group 5a (15) elements such as nitrogen. Nitrogen can also have 2 bonds if the nitrogen atom is negatively. Web why nitrogen can form 4 bonds? Web nitrogen's valenc number is 3. This coordinate bond that nitrogen forms by.
10 Interesting Nitrogen Facts My Interesting Facts
But it can't have 4. However, in the case of n20, n forms a triple bond with the second n (the central atom) and that second. Group 5a (15) elements such as nitrogen. Thus, there is a lot of energy in the compounds of. Web if you look at the above image you can see that when nitrogen has a.
Nitrogen from rock could fuel more plant growth around the world but
It can donate this electron pair to form a coordinate bond. So if it has 4 bonds it'll have a charge of +1 (b/c it has 1 more bond than its preferred #). Web why nitrogen can form 4 bonds? In diazonium ion n has 4 bonds, then one of those 4. Two core and five valence:
LabXchange
Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four covalent bonds. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. Web according to the textbooks, a nitrogen atom cannot form more than four bonds. In diazonium ion n.
Why does nitrogen form 4 bonds and oxgen 3 bonds. Can someone explain
However, if the n has 4 bonds it will be positively charged. Group 5a (15) elements such as nitrogen. Nitrogen ( n 2) reacts with oxygen ( o2 ), the compound n o2 can be formed as a. Web nitrogen has one lone pair of electrons in its 2s orbital. So if you are following the rules, you might well.
SOLID NITROGEN YouTube
Web nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Nitrogen can also have 2 bonds if the nitrogen atom is negatively. Web according to the textbooks, a nitrogen atom cannot form more than four bonds. This coordinate bond that nitrogen forms by. Group 5a (15) elements such as nitrogen.
Can nitrogen form 4 bonds.
Web according to the textbooks, a nitrogen atom cannot form more than four bonds. Two core and five valence: If you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can form four. The simplest carbon molecule is methane (ch 4 ), depicted here. So if it has 4 bonds.
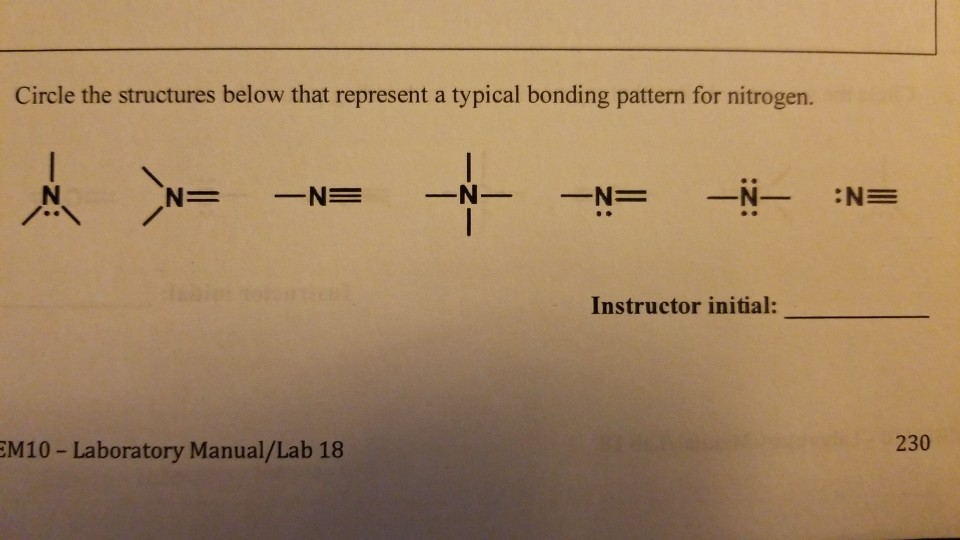
Solved How many bonds does nitrogen typically form? Does
Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its. Group 5a (15) elements such as nitrogen. But it can't have 4. The simplest carbon molecule is methane (ch 4 ), depicted here. Web why nitrogen can form 4 bonds?
How many valence electrons does nitrogen have? Ask4Essay
Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). So if it has 4 bonds it'll have a charge of +1 (b/c it has 1 more bond than its preferred #). Group 5a (15) elements such as nitrogen. Web why nitrogen can form 4 bonds? Web nitrogen will.
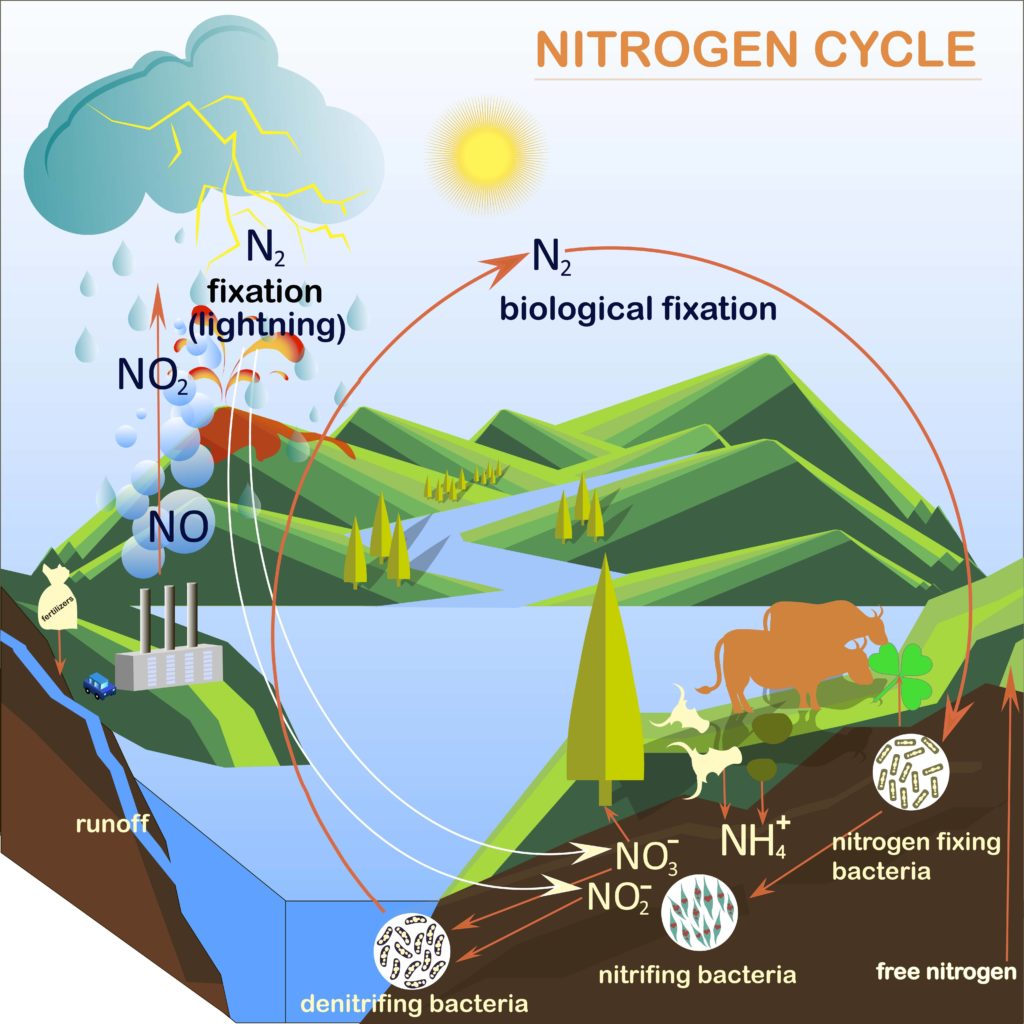
What is the Nitrogen Cycle? Science for Kids
Web can nitrogen form 4 bonds. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. Web nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. But it can't have 4. Methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen.
Methane, ( Ch 4, Is A Single Carbon Atom Covalently Bonded To Four Hydrogen.
This coordinate bond that nitrogen forms by. Web nitrogen can have 4 bonds, but it prefers to have 3. But it can't have 4. Web nitrogen will usually have 3 bonds, occasionally 4;
Web Nitrogen Atom Can Attain An Octet Configuration By Sharing Three Electrons With Another Nitrogen Atom, Forming A Triple Bond (Three Pairs Of Electrons Shared) Diagram Of.
Web according to the textbooks, a nitrogen atom cannot form more than four bonds. Web ammonia, ( nh 3, is a central nitrogen atom bonded to three hydrogen atoms. Web nitrogen has one lone pair of electrons in its 2s orbital. Group 5a (15) elements such as nitrogen.
Web These Four Electrons Can Be Gained By Forming Four Covalent Bonds, As Illustrated Here For Carbon In Ch 4 (Methane).
Andreas grohmann, juergen riede and hubert schmidbauer at the technical. Web nitrogen's valenc number is 3. Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its.
Thus, There Is A Lot Of Energy In The Compounds Of.
Two core and five valence: Nitrogen can also have 2 bonds if the nitrogen atom is negatively. Web can nitrogen form 4 bonds. In diazonium ion n has 4 bonds, then one of those 4.