Chapter 17 Thermochemistry Answer Key
Chapter 17 Thermochemistry Answer Key - Web download chapter 17 thermochemistry test answer key: The system includes the components involved. This always flows from a. All of the above ____ 7. What is chemical potential energy? How is dynamite used as kinetic energy? 1 answer in the given process the candle is losing heat as it solidifies this means that the heat is flowing out from. Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? Not all words will be used!!! Web add 2+ thermochemical equations to get a final equation, you can add the heats of reaction to get final heat of reaction.
Web science chemistry physical chemistry ch 17 thermochemistry practice test 5.0 (1 review) calorie click the card to flip 👆 quantity of heat needed to raise the temperature of 1 g of water by 1c click the. Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: How is dynamite used as kinetic energy? Web chapter 17 thermochemistry test b 67 points. This always flows from a. Not all words will be used!!! Energy stored in chemical bonds of a substance. Chapter 17 thermochemistry test answer key. A piece of metal is heated, then submerged. Web answer key chapter 17:
How is dynamite used as kinetic energy? Metric unit for heat/energy calorie: Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. When fuel is burned the products are carbon(soot) carbon monoxide and h2o in. Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. Not all words will be used!!! All chemical processes involve the transfer of heat to or from the system. The property that is useful for keeping track of heat transfers in chemical. Web terms in this set (30) thermochemistry.
Chapter 17 thermochemistry sections 17.3 & 17.4
1 answer in the given process the candle is losing heat as it solidifies this means that the heat is flowing out from. What is chemical potential energy? Web heat what is the symbol for heat? The system includes the components involved. Web answer key chapter 17:
Chem Basic FB Answer Key Ch 17 (06.14.16)
Thermochemistry 17.1 chemical potential energy review questions 1. Web terms in this set (30) thermochemistry. 11 answer in incomplete combustion; Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? Web add 2+ thermochemical equations to get a final equation, you can add the heats of.
141 Thermochemistry worksheet key Thermochemistry Worksheet Key How
Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? 11 answer in incomplete combustion; 1 answer in the given process the candle is losing heat as it solidifies this means that the heat is flowing out from. The system includes the components involved. When fuel.
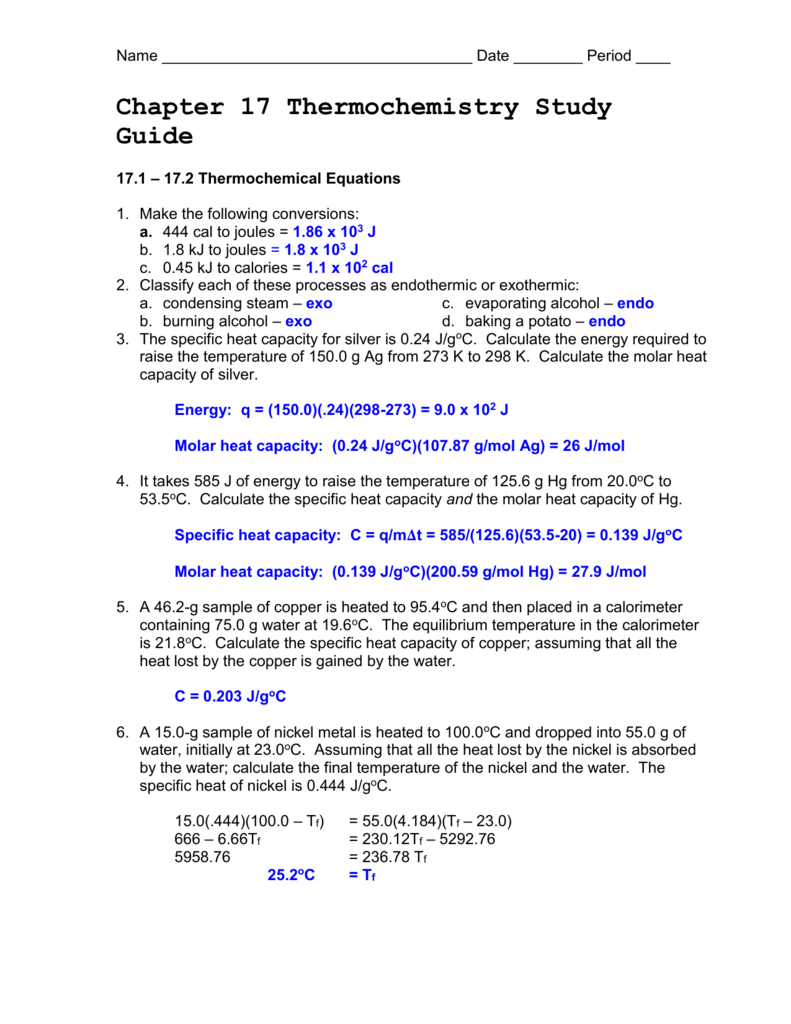
Chapter 17 Thermochemistry Study Guide
Web the amount of heat needed to increase the temperature of an object exactly 1 degree celsius. Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? Chapter 17 thermochemistry test answer key. Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. Metric unit for.
answer key thermochemistry review
Amount of energy needed to change 1 gram of water 1°c kilocalorie (calorie or food calorie) 2. The property that is useful for keeping track of heat transfers in chemical. All chemical processes involve the transfer of heat to or from the system. Web terms in this set (30) thermochemistry. How is gasoline used as kinetic energy?
Chapter 17 thermochemistry sections 17.3 & 17.4
Web answer key chapter 17: Web the amount of heat needed to increase the temperature of an object exactly 1 degree celsius. Metric unit for heat/energy calorie: The property that is useful for keeping track of heat transfers in chemical. The energy heats the parts of the engine.
Chapter 5 Thermochemistry Answers
Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? Not all words will be used!!! The system includes the components involved. The energy is lost as heat in the exhaust. Study of energy changes that occur during chemical reactions and changes in state.
11 Thermochemistry
Web chapter 17 thermochemistry test b 67 points. The energy is lost as heat in the exhaust. This always flows from a. Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers..
PPT Chapter 17 Review “Thermochemistry” PowerPoint Presentation, free
How is gasoline used as kinetic energy? Matching (2 points each) write the letter of the correct term with its description. Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? When fuel is burned the products are carbon(soot) carbon monoxide and h2o in. Web terms.
Chapter 17 thermochemistry sections 17.3 & 17.4
Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: How is dynamite used as kinetic energy? Not all words will be used!!! Energy stored in chemical bonds of a substance. Web terms in this set (30) thermochemistry.
Study Of Energy Changes That Occur During Chemical Reactions And Changes In State.
Q heat flows from the (warmer/cooler) object to a (warmer/cooler) object warmer, cooler what do chemical reactions and changes in physical state generally involve? Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: The system includes the components involved. Web chapter 17 thermochemistry test b 67 points.
Web Science Chemistry Physical Chemistry Ch 17 Thermochemistry Practice Test 5.0 (1 Review) Calorie Click The Card To Flip 👆 Quantity Of Heat Needed To Raise The Temperature Of 1 G Of Water By 1C Click The.
Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. Write the date at the top of the. How is gasoline used as kinetic energy? The amount of heat it takes to raise the temperature of 1g of the substance 1 degree celsius.
Metric Unit For Heat/Energy Calorie:
Not all words will be used!!! Thermochemistry 17.1 chemical potential energy review questions 1. The property that is useful for keeping track of heat transfers in chemical. The energy is transformed into work to move the car.
The Energy Heats The Parts Of The Engine.
Web heat what is the symbol for heat? Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. The energy is lost as heat in the exhaust. How is dynamite used as kinetic energy?