Chapter 2 The Chemistry Of Life
Chapter 2 The Chemistry Of Life - Learn vocabulary, terms, and more with flashcards, games, and other study tools. Web since organisms are made of elements arranged into molecule, the laws of physics and chemistry must apply to all biological processes. Data analysis and presentation (instructor materials preparation) 2.1: Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. In this chapter, we will explore the chemistry of how atoms are. This document was uploaded by user and they confirmed that they have the permission to share it. Web chapter 2 assessment the chemistry of life term 1 / 33 the positively charged particle in an atom is called the click the card to flip 👆 definition 1 / 33 proton click the card to flip 👆 flashcards learn test match created by. Web start studying chapter 2: Removing energy (cooling) atoms and molecules decreases their motion,. Introduction to the chemistry of life.
The chapter describes biochemical compounds and reactions as well as the significance of water to life. In this chapter, we will explore the chemistry of how atoms are. Data analysis and presentation (instructor materials preparation) 2.1: Click the card to flip 👆 definition 1 / 72 protons neutrons electrons click the card. There are 3 videos in the series: Gas, liquid, solid atoms are the. Web since organisms are made of elements arranged into molecule, the laws of physics and chemistry must apply to all biological processes. If you are author or own the copyright of this. The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? Web chapter 2 the chemistry of life study guide terms in this set (85) the main source of energy for living things carbohydrates help carry out chemical reactions proteins contain hydrogen, oxygen, nitrogen,.
Web chapter 2 assessment the chemistry of life term 1 / 33 the positively charged particle in an atom is called the click the card to flip 👆 definition 1 / 33 proton click the card to flip 👆 flashcards learn test match created by. Web this chapter provides the chemistry background needed to understand the human body, its functions, and its processes. 2 every object in the universe is composed in matter matter is anything that occupies space and has mass o found on earth in 3 physical states: Chemistry of life (instructor materials preparation) 2: The center of the atom that is formed by protons and neutrons bound together with strong forces. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and. The chapter describes biochemical compounds and reactions as well as the significance of water to life. Web the subatomic particles that make up atoms are protons, neutrons, and electrons. The video is shot in dr. In this chapter, we will explore the chemistry of how atoms are.
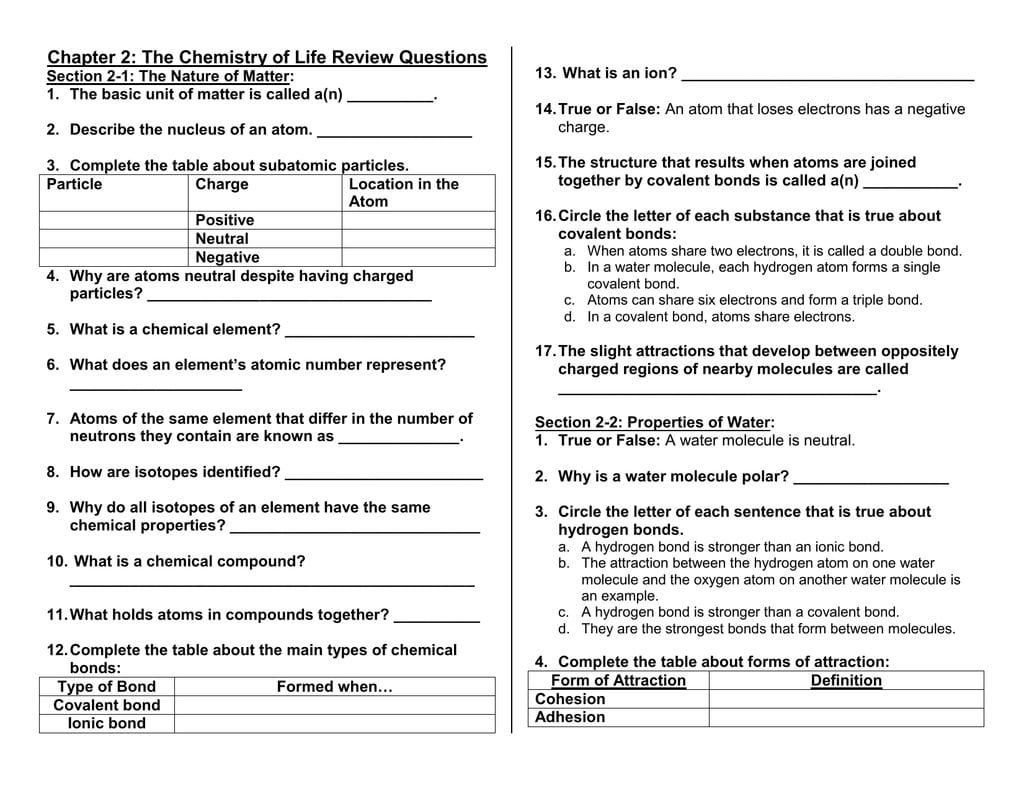
Chapter 2 The Chemistry Of Life Review Questions —
Web the subatomic particles that make up atoms are protons, neutrons, and electrons. Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. This document was uploaded by user and they confirmed that they have the permission to share it. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Web chemistry.
Biology Chapter 2 The Chemistry of Life
Water and carbon compounds play essential roles in organisms, which carry out chemical reactions in their daily life. In this chapter, we will explore the chemistry of how atoms are. The center of the atom that is formed by protons and neutrons bound together with strong forces. Web chemistry of life chapter 2. Web the subatomic particles that make up.
Chapter 2 Chemistry of Life
The basic unit of matter. Web it covers atoms, elements, subatomic particles, chemical bonds, and chemical reactions. Web chapter 2 the chemistry of life study guide terms in this set (85) the main source of energy for living things carbohydrates help carry out chemical reactions proteins contain hydrogen, oxygen, nitrogen,. 2.1 the nature of matter what 3 subatomic particles make.
PPT Chapter 2 Notes The Chemistry of Life PowerPoint Presentation
For each of the following. The center of the atom that is formed by protons and neutrons bound together with strong forces. 2.1 the nature of matter what 3 subatomic particles make up atoms? Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. Gas, liquid, solid atoms are the.
Chapter 2 The Chemistry Of Life Worksheet Answers —
Learn vocabulary, terms, and more with flashcards, games, and other study tools. The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? The chapter describes biochemical compounds and reactions as well as the significance of water to life. Web chapter 2 the chemistry of life study guide.
chapter 2 The chemistry of life Crossword WordMint
Web section summaries/chapter 2 37 name_____ class_____ date _____ four groups of organic compounds found in living things are carbohydrates, lipids, nucleic acids, and proteins. The video is shot in dr. Figure 2.0 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. The center of the atom that is formed by protons and neutrons bound together.
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
Water and carbon compounds play essential roles in organisms, which carry out chemical reactions in their daily life. Click the card to flip 👆 definition 1 / 72 protons neutrons electrons click the card. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and. Web this chapter provides the chemistry background needed to understand the human body, its functions, and its processes..
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
Web this chapter provides the chemistry background needed to understand the human body, its functions, and its processes. There are 3 videos in the series: Gas, liquid, solid atoms are the. Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. Web chemistry of life chapter 2.
PPT Chapter 2 Chemistry of Life PowerPoint Presentation, free
Web since organisms are made of elements arranged into molecule, the laws of physics and chemistry must apply to all biological processes. There are 3 videos in the series: Web the chemistry of life (chapter 2) chemical bonds join together the molecules and compounds of life. Web start studying chapter 2: 2.1 the nature of matter what 3 subatomic particles.
PPT Chapter 2 Chemistry of Life PowerPoint Presentation, free
The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? For each of the following. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Click the card to flip 👆 definition 1 / 72 protons neutrons electrons click the card. In this chapter, we.
Web Chapter 2 The Chemistry Of Life Study Guide Terms In This Set (85) The Main Source Of Energy For Living Things Carbohydrates Help Carry Out Chemical Reactions Proteins Contain Hydrogen, Oxygen, Nitrogen,.
Learn vocabulary, terms, and more with flashcards, games, and other study tools. What are the basic chemical principles that affect living things? Web the subatomic particles that make up atoms are protons, neutrons, and electrons. Web it covers atoms, elements, subatomic particles, chemical bonds, and chemical reactions.
In Your Textbook, Read About The Role Of Carbon.
Chemistry of life (instructor materials preparation) 2: Gas, liquid, solid atoms are the. Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. 2 every object in the universe is composed in matter matter is anything that occupies space and has mass o found on earth in 3 physical states:
Data Analysis And Presentation (Instructor Materials Preparation) 2.1:
Web chemistry of life chapter 2. The video is shot in dr. Removing energy (cooling) atoms and molecules decreases their motion,. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and.
This Document Was Uploaded By User And They Confirmed That They Have The Permission To Share It.
Web since organisms are made of elements arranged into molecule, the laws of physics and chemistry must apply to all biological processes. If you are author or own the copyright of this. Introduction to the chemistry of life. 2.1 the nature of matter what 3 subatomic particles make up atoms?