Chapter 5 Electrons In Atoms
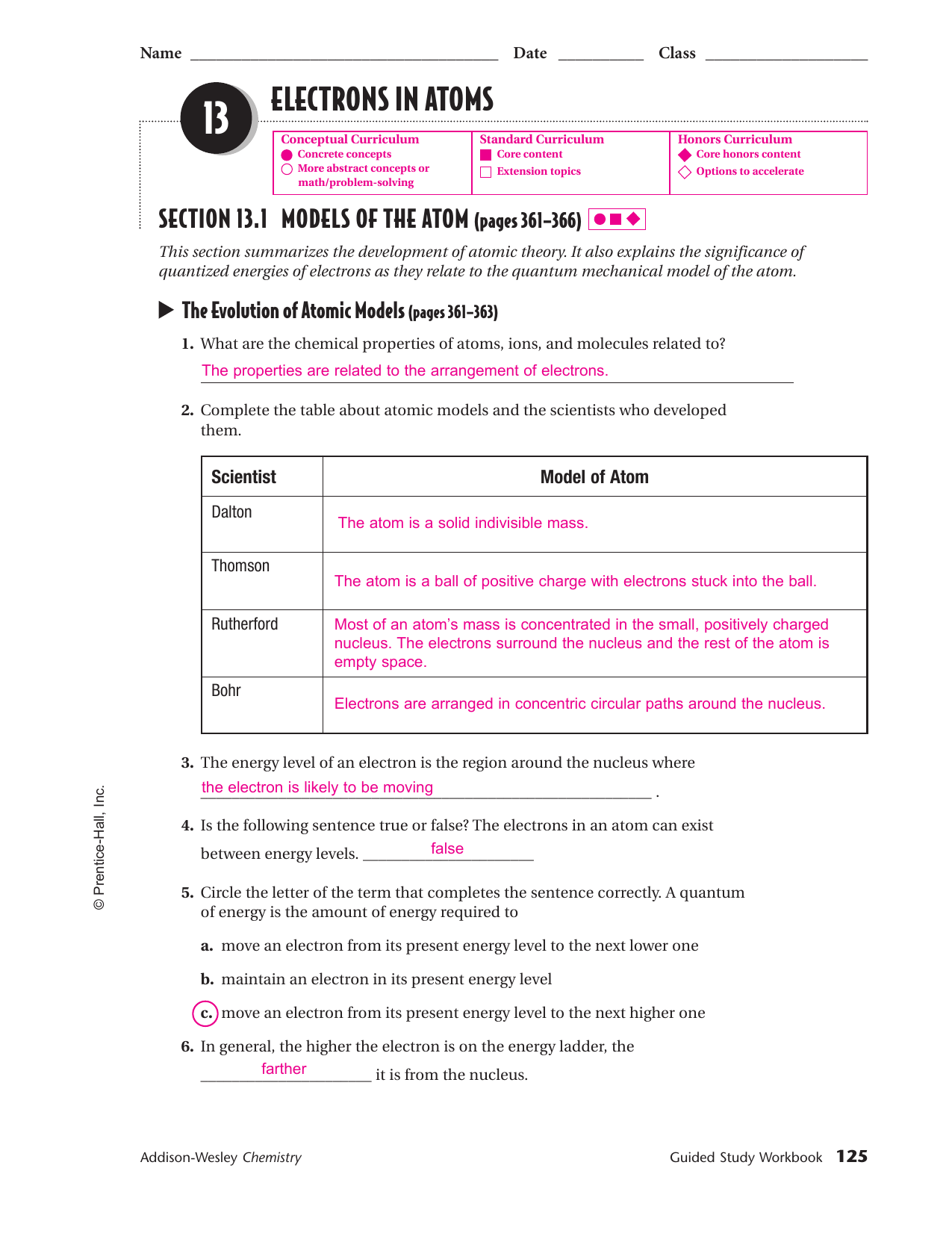
Chapter 5 Electrons In Atoms - Tendency of electrons to enter orbitals of lowest energy first. Web each shell can hold two electrons which allow orbital p to hold six electrons, orbital d can hold ten electrons, and orbital f can hold fourteen electrons. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy. Web the quantum mechanical model of the atom. 1s1, 2s2, 2p2, 3s2, 3p3, 4s2. Objectives compare the wave and particle natures of light. The energy levels contained within a principle energy level. Chapter 5 “electrons in atoms” section 5.1 models of the atom objectives: • identify the inadequacies in the rutherford atomic model. It also explains the significance of quantized energies of electrons.
Click the card to flip 👆 definition 1 / 51 ground state: Is concerned with the probability of finding an electron in a certain position. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. • identify the inadequacies in the rutherford atomic model. Terms in this set (18) energy levels. This arrangement of electrons is the most ___ arrangement and is the atoms. Each of these orbits has a specific number of electrons. The ratio of the number of observations in a statistical. Define a quantum of energy, and.
Tendency of electrons to enter orbitals of lowest energy first. Is concerned with the probability of finding an electron in a certain position. The maximum number of electrons that. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy. Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. The ratio of the number of observations in a statistical. Electrons can jump from one level to another when excited (from the ground state to an excited state). In the second period elements, the two electrons in the 1s sublevel are called. Web the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms. The arrangement of electrons in a atom's ___.
Electrons in Atoms
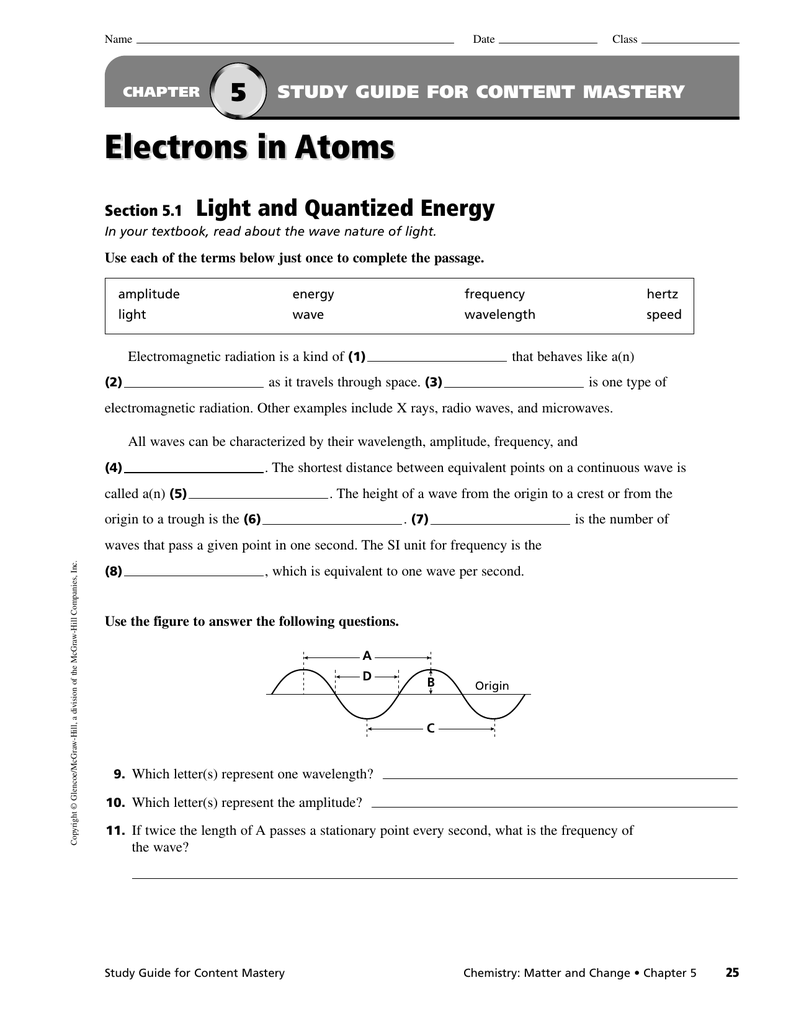
Web chapter5 what you’ll learn you will compare the wave and particle models of light. Web the height of a wave from the origin to a crest, or from the origin to the trough. This arrangement of electrons is the most ___ arrangement and is the atoms. Terms in this set (18) energy levels. The arrangement of electrons in a.
Chapter 5 electrons in atoms
Web the height of a wave from the origin to a crest, or from the origin to the trough. Web the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms. You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. Click the.
Chapter 5 Electrons In Atoms Answer Key THE PREMIER FEMALE DJ OF LOS
Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. Web what are the electrons doing and how do they move ? You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. Is concerned with the probability of finding an electron in a certain position. Web in this.
Chemistry Chapter 5 Electrons In Atoms Study Guide Answers Study Poster
• identify the inadequacies in the rutherford atomic model. Define a quantum of energy, and. Each of these orbits has a specific number of electrons. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? Arrangement of electrons around atomic nucleus.
10+ Chapter 5 Electrons In Atoms Answer Key WilliamMiran
Chapter 5 “electrons in atoms” section 5.1 models of the atom objectives: Electrons can jump from one level to another when excited (from the ground state to an excited state). Web the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms. • identify the inadequacies in the rutherford atomic model. It.
Chemistry Chp 5 Electrons In Atoms Powerpoint
Chapter 5 “electrons in atoms” section 5.1 models of the atom objectives: Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. This arrangement of electrons is the most ___ arrangement and is the atoms. Each of these orbits has a specific number of electrons. Define a quantum of energy, and.
Chapter 5 Electrons in Atoms
Valence electrons are the electrons in the highest occupied principal energy level of an atom. Web in this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to their energies. Arrangement of electrons around atomic nucleus. Electrons in an atom tend to assume the arrangement that gives the atom the ___.
Chapter 5 electrons in atoms
The rule that electrons occupy the orbitals of lowest energy first. • identify the inadequacies in the rutherford atomic model. You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. The arrangement of electrons in a atom's ___. Electrons exist when they're not excited.
Chapter 5 electrons in atoms
The maximum number of electrons that. Electrons can jump from one level to another when excited (from the ground state to an excited state). Electrons exist when they're not excited. Each of these orbits has a specific number of electrons. Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength.
Chapter 5 Electrons in Atoms
Web in this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to their energies. Web the quantum mechanical model of the atom. Is concerned with the probability of finding an electron in a certain position. It also explains the significance of quantized energies of electrons. 1s1, 2s2, 2p2, 3s2, 3p3,.
Valence Electrons Are The Electrons In The Highest Occupied Principal Energy Level Of An Atom.
Arrangement of electrons around atomic nucleus. Electrons exist when they're not excited. Web what are the electrons doing and how do they move ? Is concerned with the probability of finding an electron in a certain position.
• Identify The Inadequacies In The Rutherford Atomic Model.
Each of these orbits has a specific number of electrons. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy. Web the quantum mechanical model of the atom. 4 answer by gaining or losing certain amounts of energy, electrons can make quantum jumps.
Web The Height Of A Wave From The Origin To A Crest, Or From The Origin To The Trough.
The rule that electrons occupy the orbitals of lowest energy first. Diagrams that show valence electrons as dots. Click the card to flip 👆 definition 1 / 51 ground state: Web chapter5 what you’ll learn you will compare the wave and particle models of light.
We Also Explain How Knowing The Arrangement Of Electrons In.
Objectives compare the wave and particle natures of light. 1s1, 2s2, 2p2, 3s2, 3p3, 4s2. Web electrons exist in fixed pathways (orbits) around the nucleus. The maximum number of electrons that.