Chapter 6 The Periodic Table And Periodic Law
Chapter 6 The Periodic Table And Periodic Law - Web chapter 6 the periodic table and periodic law section 6.1 development of the pt 1. He discovered that the properties of elements repeat in a periodic way. Web the number of protons in the nucleus of an atom. Protons in the nucleus of an atom of the element. Explain why elements in a group have similar properties. Relate the group and period trends seen in. The periodic table and periodic law. The periodic table and periodic law in this chapter: That when elements are arranged according to increasing atomic number there is a periodic repetition of their chemical and physical properties. Web tjdubinski terms in this set (26) periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their properties.
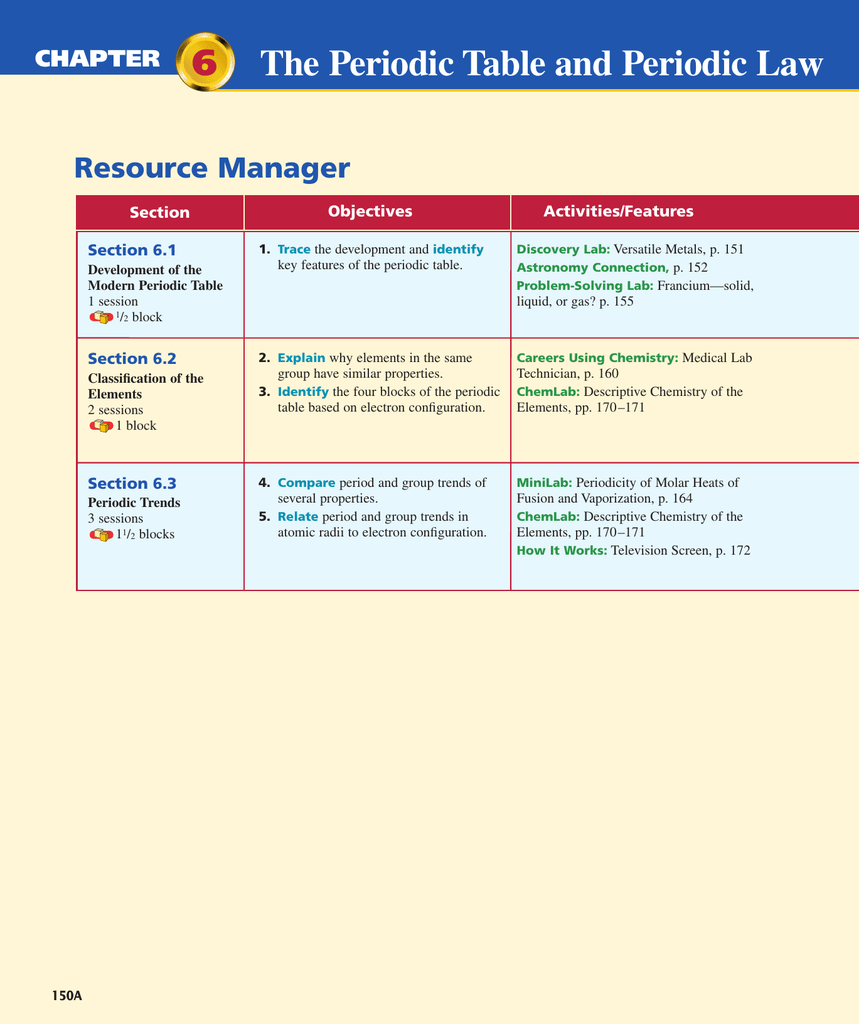
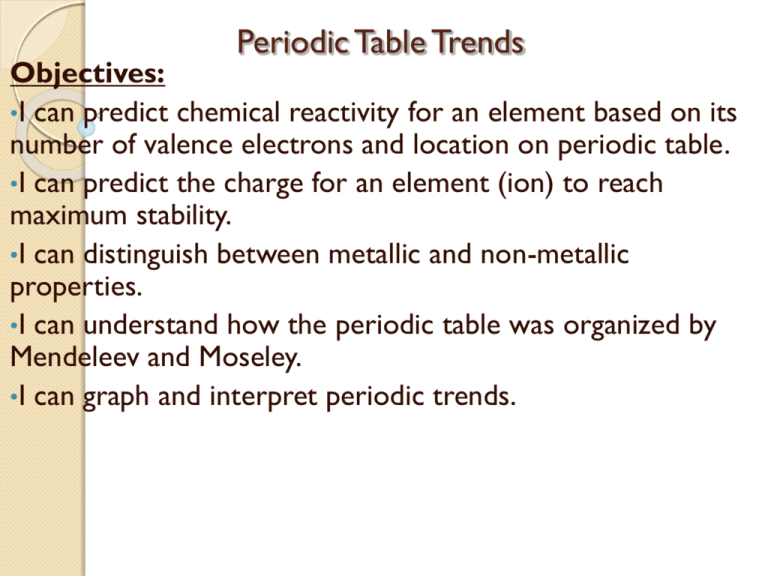
Web problem 1 describe the development of the modern periodic table. The arrangement of the periodic table is based on the number of: Relate the group and period trends seen in. The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. A column on the periodic table. When interpreted properly, the table describes much of the elements physical. Include contributions made by lavoisier, newlands, mendeleev, and moseley. Explain why elements in a group have similar properties. Identify different key features of the periodic table. Web chapter 6 the periodic table and periodic law vocabulary.
The periodic table and periodic law. Properties of groups and periods in the late 1800s, russian chemist dmitri mendeleev created the periodic table by organizing elements by their atomic weight in increasing. The periodic table and periodic law. Protons in the nucleus of an atom of the element. Web the number of protons in the nucleus of an atom. Web chapter 6 the periodic table and periodic law vocabulary. Relate the group and period trends seen in. What did newlands discovered (1865)? The periodic table and periodic law in this chapter: Web start studying chapter 6:
Chemistry Chapter 6 The Periodic Table Worksheet Answers
Group 1 a elements, except for hydrogen, that are on the left side of the modern periodic. Identify different key features of the periodic table. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. Web chapter 6 the periodic table and periodic law section 6.1 development of the pt 1. Web problem.
Chapter 6 Study Guide The Periodic Table And Periodic Law Study Poster
The periodic table and periodic law. Web start studying chapter 6: Web increasing atomic number is called the periodic law. A column on the periodic table. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Chapter 6 The Periodic Table And Periodic Law Study Guide Study Poster
Learn vocabulary, terms, and more with flashcards, games, and other study tools. The periodic table and periodic law in this chapter: When interpreted properly, the table describes much of the elements physical. What did newlands discovered (1865)? A column on the periodic table.
Chapter 6 The Periodic Table and Periodic Law
Explain why elements in a group have similar properties. The arrangement of the periodic table is based on the number of: Reading check compare and contrast the ways in which mendeleev and moseley organized the elements. Representative elements group a elements transition elements group b elements The periodic table and periodic law.
Chapter 6 Periodic table
Lilia pronin numerade educator 01:12 problem 2 sketch a simplified. Group 1 a elements, except for hydrogen, that are on the left side of the modern periodic. The periodic table and periodic law in this chapter: The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. Web video.
Chapter 6 Periodic Table CeilidhMavi
Web tjdubinski terms in this set (26) periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their properties. Group 1 a elements, except for hydrogen, that are on the left side of the modern periodic. Table 6.2 summarizes the contributions of. That when elements are arranged according to increasing atomic.
Chapter 6 The Periodic Table and Periodic Law
He discovered that the properties of elements repeat in a periodic way. Properties of groups and periods in the late 1800s, russian chemist dmitri mendeleev created the periodic table by organizing elements by their atomic weight in increasing. Web the number of protons in the nucleus of an atom. Table 6.2 summarizes the contributions of. Web start studying chapter 6:
Chapter 6 the periodic table
Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. What did newlands discovered (1865)? Properties of groups and periods in the late 1800s, russian chemist dmitri mendeleev created the periodic table by organizing elements by their atomic weight in increasing. The arrangement of the periodic table is based on the number of:.
Chemistry Chapter 6 The Periodic Table Worksheet Answers
The periodic table and periodic law. Web periodic law (6.1) statement that there is a periodic repetition of chemical and physical properties of the elements when arranged by increasing atomic number. Web 1/16 previous ← next → flip space created by ambssbabyy123 chemistry test review terms in this set (16) periodic law statement that when the elements are arranged by.
Chapter 6 The Periodic Table and Periodic Law
Relate the group and period trends seen in. That when elements are arranged according to increasing atomic number there is a periodic repetition of their chemical and physical properties. When interpreted properly, the table describes much of the elements physical. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. Web increasing atomic.
When The Elements Are Arranged In Order Of Increasing Atomic Number, There Is A Periodic Pattern In Their Physical And Chemical Properties.
Web video answers for all textbook questions of chapter 6, the periodic table and periodic law, glencoe chemistry by numerade Lilia pronin numerade educator 01:12 problem 2 sketch a simplified. That when elements are arranged according to increasing atomic number there is a periodic repetition of their chemical and physical properties. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;.
Web Start Studying Chapter 6:
Reading check compare and contrast the ways in which mendeleev and moseley organized the elements. The periodic table and periodic law in this chapter: Web chapter 6 the periodic table and periodic law section 6.1 development of the pt 1. Table 6.2 summarizes the contributions of.
Web Periodic Law (6.1) Statement That There Is A Periodic Repetition Of Chemical And Physical Properties Of The Elements When Arranged By Increasing Atomic Number.
Properties of groups and periods in the late 1800s, russian chemist dmitri mendeleev created the periodic table by organizing elements by their atomic weight in increasing. The periodic table and periodic law. Relate the group and period trends seen in. Click the card to flip 👆.
What Did Newlands Discovered (1865)?
The arrangement of the periodic table is based on the number of: Representative elements group a elements transition elements group b elements Group 1 a elements, except for hydrogen, that are on the left side of the modern periodic. Identify different key features of the periodic table.