Chapter 7 Chemical Formulas And Chemical Compounds Answer Key
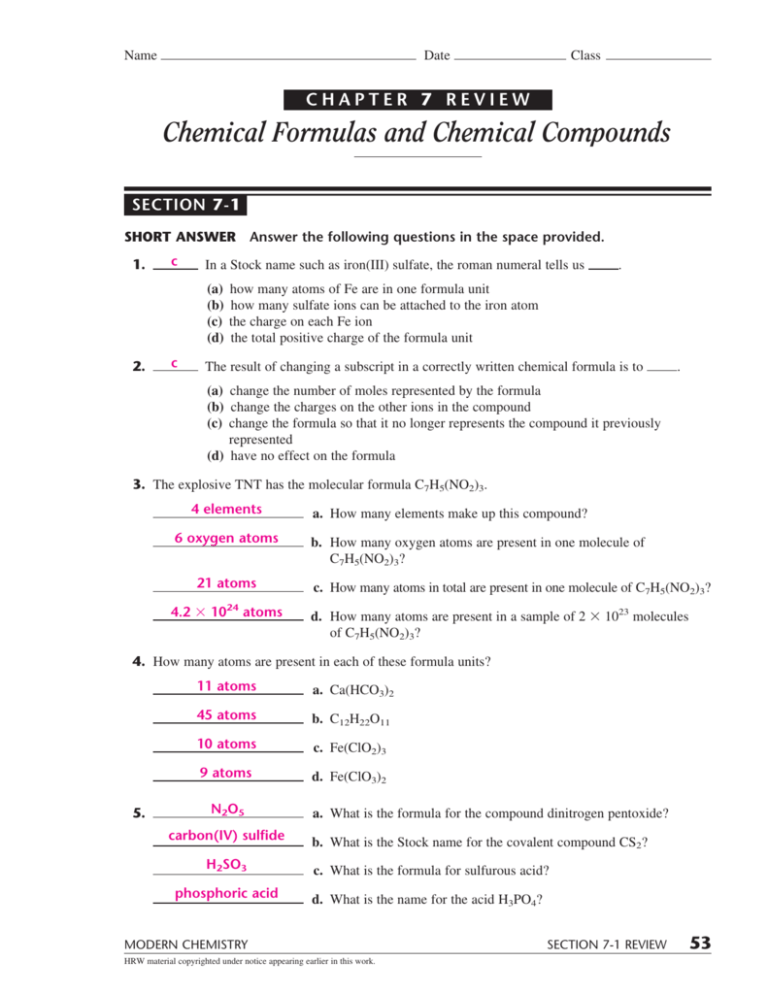
Chapter 7 Chemical Formulas And Chemical Compounds Answer Key - Web adv chem resource nearpod codes chapter seven: Ions with greater number of oxygen atoms ends in. Various rules are used to name ionic and covalent compounds. Polyatomic anions that contain oxygen. Chemical nomenclature 7.1 ionic compounds lesson review questions questions 1. Web chapter 7 review chemical formulas and chemical compounds section 4 short answer answer the following questions in the space provided. Positive charges form when electrons are lost. Web big idea chemical formulas represent the ratios of atoms in a chemical compound. Web chapter 7 practice test with answer key.pdf. What is the purpose of an empirical formula?
What is the purpose of an empirical formula? For each of the following ionic compounds… Write empirical formulas to match the following molecular. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Determine the smallest angular velocity of the shaft if it is restricted not to twist more than 1°. Bonds form between the atoms. Hydrocarbon (molecule with only h and c ). Atomic mass of each element. Number of atoms of each element in a single. It is required to transmit 35 kw of power from the engine e to the generator g.
C) type of bond holding particles together in a compound. Ions with greater number of oxygen atoms ends in. Write empirical formulas to match the following molecular. Web big idea chemical formulas represent the ratios of atoms in a chemical compound. Various rules are used to name ionic and covalent compounds. Define binary and ternary ionic compounds. Ionic compounds are composed to elements in an _______________ bond. Atomic and mass number, avogadro number and mole, branches of chemistry, chemical calculations, elements and compounds particles, elements compounds and mixtures, empirical and molecular formulas, gram atomic mass molecular mass and gram formula… The protons in the nucleus do not change during normal chemical reactions. Number of atoms of each element in a single.
Writing formulas Ionic Compounds Chem Worksheet 8 3 Answer Key
Determine the smallest angular velocity of the shaft if it is restricted not to twist more than 1°. The protons in the nucleus do not change during normal chemical reactions. Web chapter 7 chemical formulas and chemical compounds test answer key at the end of this section, you will be able to: Web chemistry mcq pdf book with answers, test.
Worksheet Chemical Formula Writing Worksheet Worksheet Ionic —
Number of atoms or ions of each element that are combined in the compound. What is the purpose of an empirical formula? What is the difference between a monatomic and a polyatomic ion? Web in chapter 7, students will learn how to write the chemical formula of any given compound, either ionic or molecular, given that compound's scientific name (or.
Chapter 7 Section 3 Using Chemical Formulas
Chemical compounds and chemical formulas chapter interactives: Click the card to flip 👆. P, i, cl, and o would form. The protons in the nucleus do not change during normal chemical reactions. Web chapter 7 practice test with answer key.pdf.
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
Ions with greater number of oxygen atoms ends in. Web a) charge on an ion. Ionic compounds are composed to elements in an _______________ bond. It is required to transmit 35 kw of power from the engine e to the generator g. Click the card to flip 👆.
Names and formulas of ionic compounds study guide
Define binary and ternary ionic compounds. Web the number of atoms of each element contained in a single molecule of the compound. For each of the following ionic compounds… Determine the smallest angular velocity of the shaft if it is restricted not to twist more than 1°. Bonds form between the atoms.
Chapter 7 Chemical Formulas And Chemical Compounds Answer Key
Define binary and ternary ionic compounds. Atomic mass of each element. Chemical nomenclature 7.1 ionic compounds lesson review questions questions 1. Chemical compounds and chemical formulas chapter interactives: Web big idea chemical formulas represent the ratios of atoms in a chemical compound.
Chemical Formulas and Chemical Compounds
Web chemistry mcq pdf book with answers, test 3 to solve mcq questions: Polyatomic anions that contain oxygen. Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. Web chapter 7 chemical formulas and chemical compounds test answer key at the end.
PPT Modern Chemistry Chapter 7 Chemical Formulas & Chemical Compounds
As 2 o 5 c. Web chapter 7 chapter 7 highlights 1. A lattice of positive and negative ions held together by mutual attraction. Various rules are used to name ionic and covalent compounds. Web the number of atoms of each element contained in a single molecule of the compound.
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Write empirical formulas to match the following molecular. Web in chapter 7, students will learn how to write the chemical formula of any given compound, either ionic or molecular, given that compound's scientific name (or vice versa). Web chemistry chapter 7 review (names and formulas) term. Covalent compounds naming and review practice with answer key… Web big idea chemical formulas.
Chapter 7.3 Using Chemical Formulas
Web adv chem resource nearpod codes chapter seven: Ionic compounds are composed to elements in an _______________ bond. For each of the following ionic compounds… Chemical formula writing worksheet review with answer key.pdf. Positive charges form when electrons are lost.
Hydrocarbon (Molecule With Only H And C ).
Number of atoms or ions of each element that are combined in the compound. Common chemicals and their common names naming ionic and covalent compounds oxidation numbers oxidation numbers #2 valence electrons and oxidation state oxidation numbers molecular and empirical formulas Positive charges form when electrons are lost. What is the purpose of an empirical formula?
Chemical Formula Writing Worksheet Review With Answer Key.pdf.
Ions with greater number of oxygen atoms ends in. Web adv chem resource nearpod codes chapter seven: D) distribution of electrons among the bonded particles in a compound. Web big idea chemical formulas represent the ratios of atoms in a chemical compound.
Write Empirical Formulas To Match The Following Molecular.
A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Web a) charge on an ion. For each of the following ionic compounds… Inorganic compounds that take the form of discrete molecules.
Web Chapter 7 Chapter 7 Highlights 1.
Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. Web chemistry chapter 7 review (names and formulas) term. B) number of atoms or ions in a compound. Chemical compounds and chemical formulas chapter interactives: