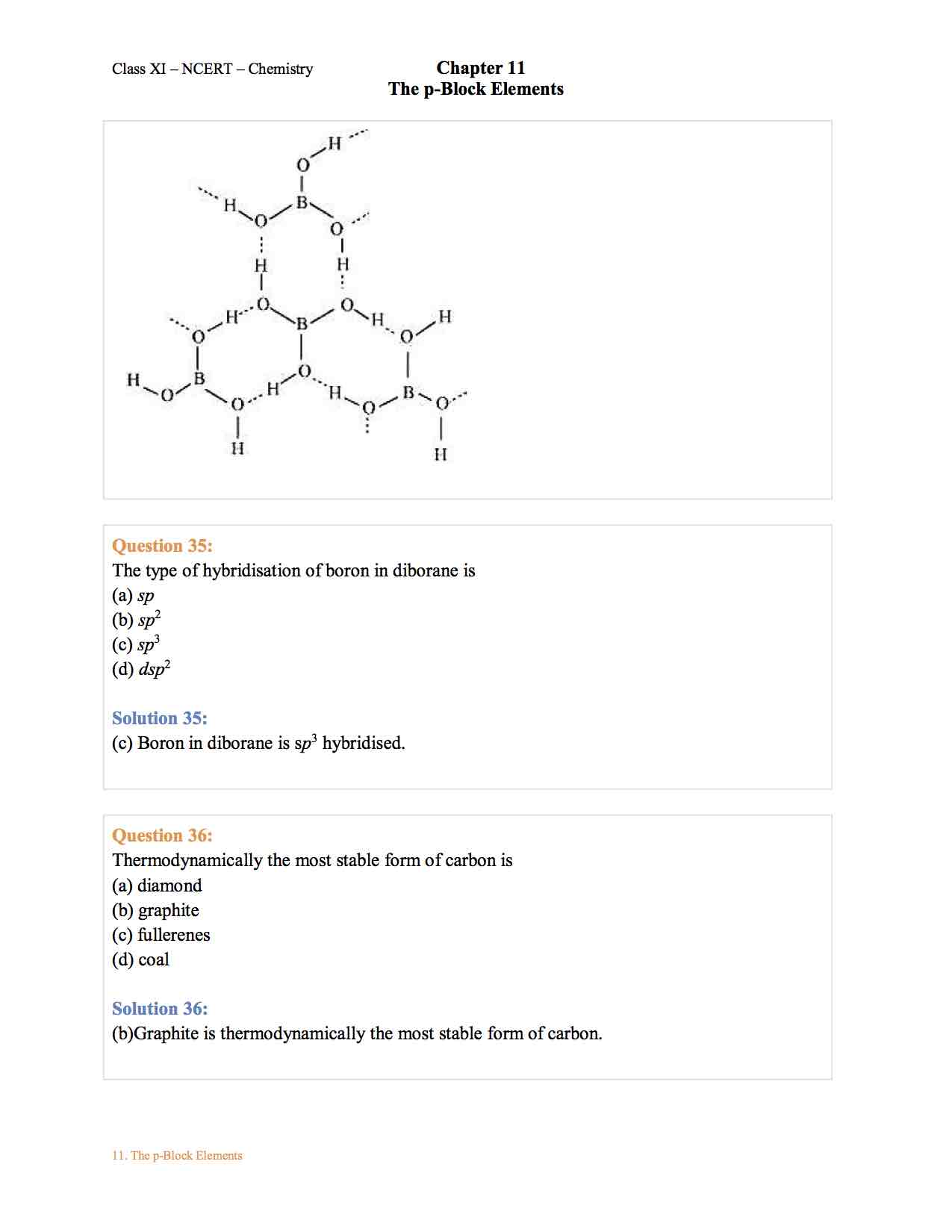
Chemistry Chapter 11
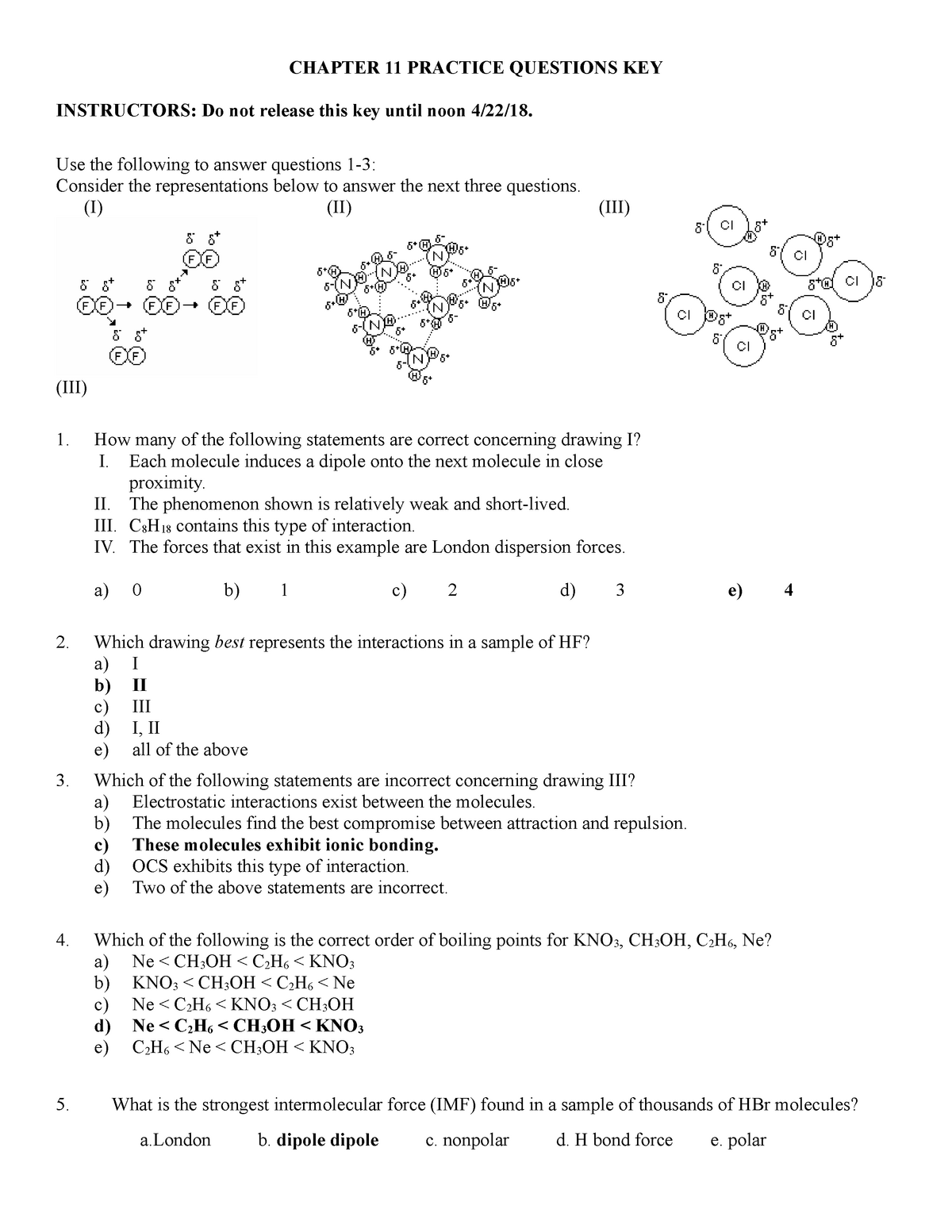
Chemistry Chapter 11 - Our solutions are written by chegg experts so you can be assured of the highest quality! You know that you have a balanced chemical equation when the. Web 18.11 occurrence, preparation, and properties of halogens; Solids, liquids and intermolecular forces 10.6: Our resource for chemistry 11 includes answers to chapter exercises, as well as detailed. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: After studying this chapter about alcohol, phenols and ethers using the ncert solutions,. Click the card to flip 👆 false click the card to flip 👆 1 / 44 flashcards learn test match. Intermolecular forces topic hierarchy chapter 11. What is the predominant intermolecular force in the liquid state of each of these compounds:
Click the card to flip 👆 false click the card to flip 👆 1 / 44 flashcards learn test match. A solution can vary in composition, while a compound cannot vary in composition. Our solutions are written by chegg experts so you can be assured of the highest quality! Our resource for chemistry 11 includes answers to chapter exercises, as well as detailed. Web the use of ncert books class 11 chemistry is not only suitable for studying the regular syllabus of various boards but it can also be useful for the candidates appearing for various competitive exams, engineering entrance exams, and olympiads. This 11th grade chemistry textbook replacement course covers all of the topics in a standard 11th grade chemistry textbook. Web position of an element in the periodic table relating to its chemical reactivity. Hydrogen fluoride (hf), carbon tetrachloride (ccl4), and. Intermolecular forces topic hierarchy chapter 11. Web this chapter considers the nature of solutions and examines factors that determine whether a solution will form and what properties it may have.
Web learn chemistry chapter 11 with free interactive flashcards. Students may follow the links on the subtopics to access free study material on the associated concepts (prepared by chemistry. (c) nonconductive (solute is a covalent compound, neither acid nor base, unreactive towards water); Web chapter 11 mastering chemistry 4.5 (4 reviews) 1. Our resource for chemistry 11 includes answers to chapter exercises, as well as detailed. (b) high conductivity (solute is a strong acid and will ionize completely when dissolved); Coefficients of the reactants equal the coefficients of the products. Click the card to flip 👆 false click the card to flip 👆 1 / 44 flashcards learn test match. Intermolecular forces topic hierarchy chapter 11. Number of atoms of each element in the reactants.
Ncert Solution for 11 Class Chemistry Chapter 11 pBlock Elements
Coefficients of the reactants equal the coefficients of the products. Web 18.11 occurrence, preparation, and properties of halogens; Students may follow the links on the subtopics to access free study material on the associated concepts (prepared by chemistry. 18.12 occurrence, preparation, and properties of the noble gases; (b) high conductivity (solute is a strong acid and will ionize completely when.
Ncert Solution for 11 Class Chemistry Chapter 1 Some Basic Concepts
The lessons offer a convenient way for students to. Web the use of ncert books class 11 chemistry is not only suitable for studying the regular syllabus of various boards but it can also be useful for the candidates appearing for various competitive exams, engineering entrance exams, and olympiads. (b) high conductivity (solute is a strong acid and will ionize.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 P Block Elements
Coefficients of the reactants equal the coefficients of the products. Our solutions are written by chegg experts so you can be assured of the highest quality! More about atoms moles and molar mass isotopes unit 3: Choose from 5,000 different sets of chemistry chapter 11 flashcards on quizlet. Web (a) high conductivity (solute is an ionic compound that will dissociate.
Class 11 Chemistry Sample Paper CBSE Papers Free PDF Download
18.12 occurrence, preparation, and properties of the noble gases; Web this class 11 chemistry index page contains all the topics that fall under each chapter of the class 11 chemistry syllabus as per the ncert textbook. Mass spectrometry mass spectrometry unit 5: The lessons offer a convenient way for students to. Web 18.11 occurrence, preparation, and properties of halogens;
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols And
Intermolecular forces topic hierarchy chapter 11. (b) high conductivity (solute is a strong acid and will ionize completely when dissolved); This 11th grade chemistry textbook replacement course covers all of the topics in a standard 11th grade chemistry textbook. Web the use of ncert books class 11 chemistry is not only suitable for studying the regular syllabus of various boards.
Chemistry Chapter 11 Stoichiometry Study Guide Answers Bestseller
Web chapter 11 mastering chemistry 4.5 (4 reviews) 1. Solids, liquids and intermolecular forces 10.6: Web this class 11 chemistry index page contains all the topics that fall under each chapter of the class 11 chemistry syllabus as per the ncert textbook. Mass spectrometry mass spectrometry unit 5: (b) high conductivity (solute is a strong acid and will ionize completely.
Class 11 Chemistry Multiple Choice Questions Part 1
Solids, liquids and intermolecular forces 10.6: The lessons offer a convenient way for students to. A solution can vary in composition, while a compound cannot vary in composition. Hydrogen fluoride (hf), carbon tetrachloride (ccl4), and. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2:
Chapter 11 Review Chemistry MarieaArshad
More about molecular composition pure substances mixtures unit 4: Hydrogen fluoride (hf), carbon tetrachloride (ccl4), and. The solvent is the most concentrated. Web access general chemistry 11th edition chapter 11 solutions now. The properties of colloids—mixtures containing dispersed.
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols And
Web position of an element in the periodic table relating to its chemical reactivity. Coefficients of the reactants equal the coefficients of the products. Our solutions are written by chegg experts so you can be assured of the highest quality! You know that you have a balanced chemical equation when the. The lessons offer a convenient way for students to.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 P Block Elements
A solution can vary in composition, while a compound cannot vary in composition. Mass spectrometry mass spectrometry unit 5: Web position of an element in the periodic table relating to its chemical reactivity. Web (a) high conductivity (solute is an ionic compound that will dissociate when dissolved); Intermolecular forces topic hierarchy chapter 11.
18.12 Occurrence, Preparation, And Properties Of The Noble Gases;
Click the card to flip 👆 false click the card to flip 👆 1 / 44 flashcards learn test match. Hydrogen fluoride (hf), carbon tetrachloride (ccl4), and. After studying this chapter about alcohol, phenols and ethers using the ncert solutions,. A solution can vary in composition, while a compound cannot vary in composition.
Solutions Are Homogeneous At The Molecular Level, While Other Mixtures.
The properties of colloids—mixtures containing dispersed. Web 18.11 occurrence, preparation, and properties of halogens; Web position of an element in the periodic table relating to its chemical reactivity. (b) high conductivity (solute is a strong acid and will ionize completely when dissolved);
Aqueous Solution (A Substances Dissolved In Water) What Is A Catalyst?
What is the predominant intermolecular force in the liquid state of each of these compounds: More about atoms moles and molar mass isotopes unit 3: Web 11.1 the dissolution process a solution forms when two or more substances combine physically to yield a mixture that is homogeneous at the molecular level. Web learn chemistry chapter 11 with free interactive flashcards.
Web This Class 11 Chemistry Index Page Contains All The Topics That Fall Under Each Chapter Of The Class 11 Chemistry Syllabus As Per The Ncert Textbook.
More about molecular composition pure substances mixtures unit 4: Our solutions are written by chegg experts so you can be assured of the highest quality! You know that you have a balanced chemical equation when the. (c) nonconductive (solute is a covalent compound, neither acid nor base, unreactive towards water);