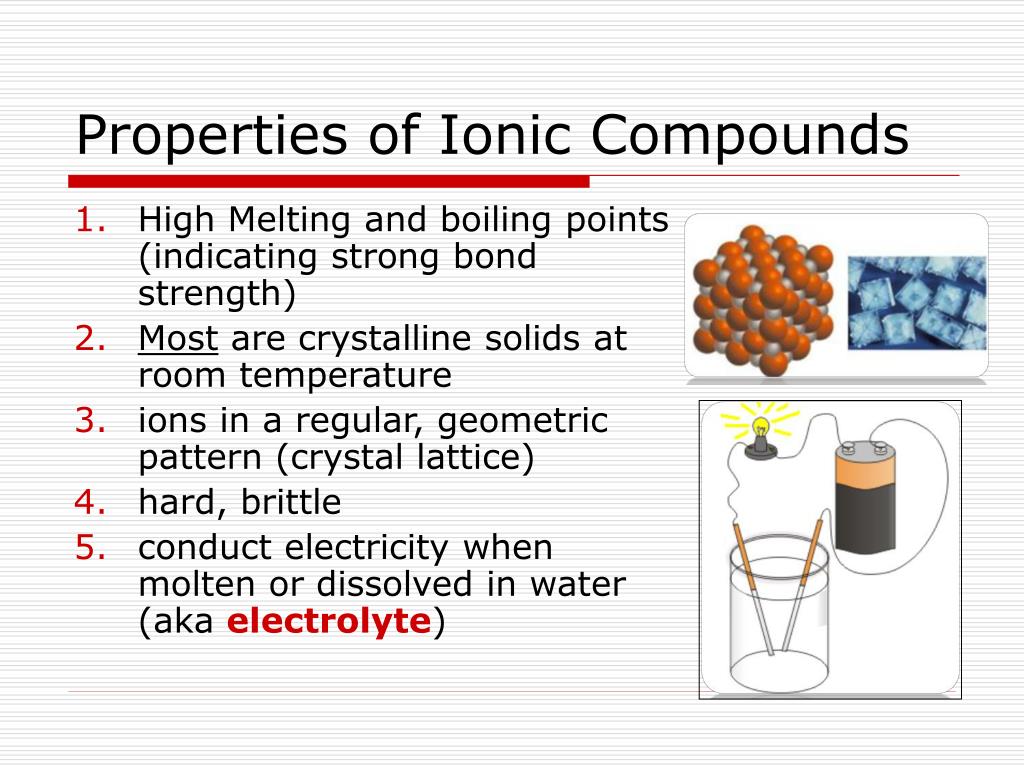
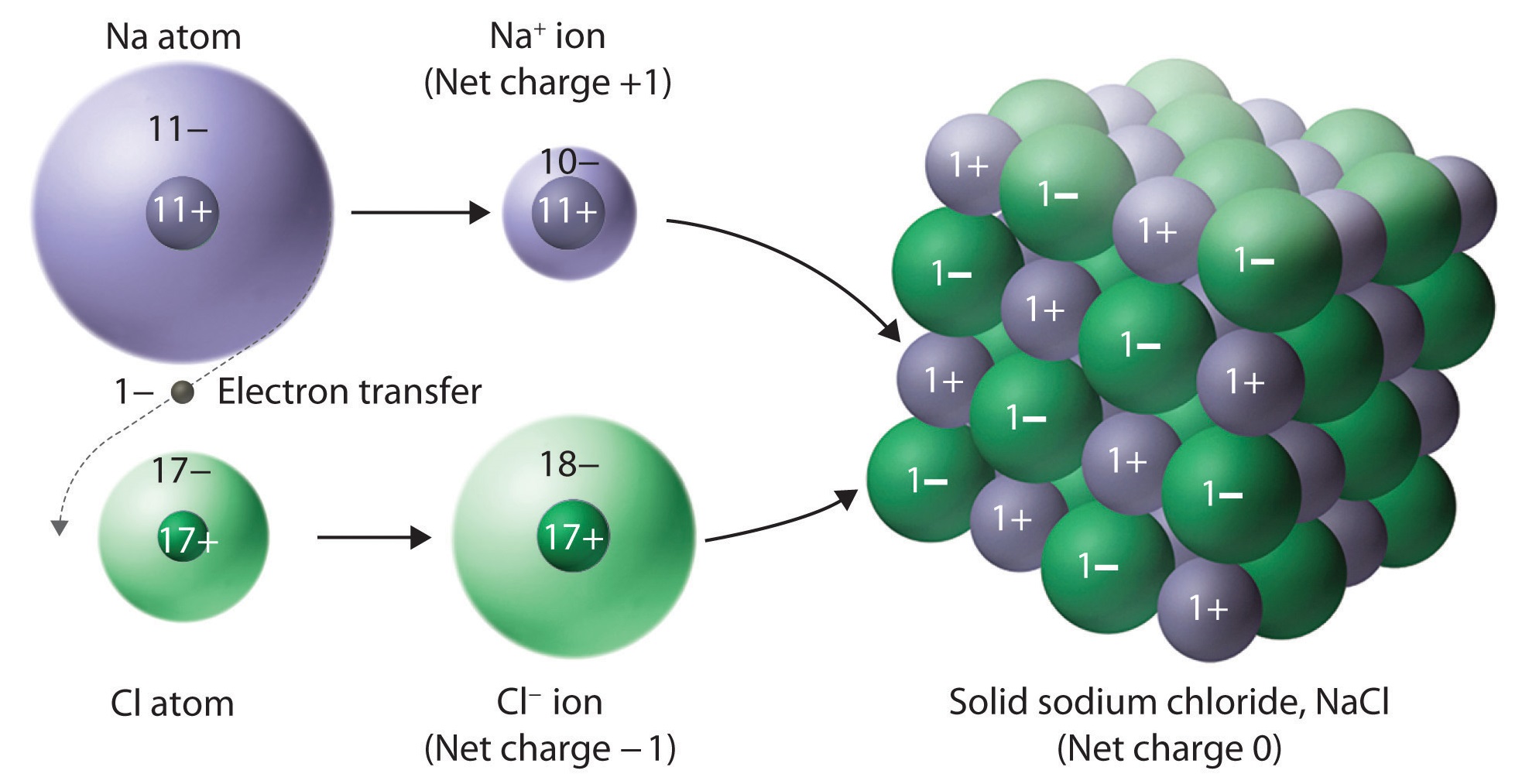
Describe How Ionic Compounds Form Crystals
Describe How Ionic Compounds Form Crystals - Web chemistry chemical bonding and molecular structure formation of ionic compounds ionic compounds what is ionic compound? Covalent crystals are hard, frequently. They can be of a single crystal or powdered form. Sheri neva / getty images. Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. Web ionic crystals are aggregates of charged ions. Ionic compounds can be defined as:. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. The opposite charges cancel out so ionic compounds have a net neutral. The arrangement maximizes the attractive force between oppositely.
Nacl or salt is an example of an ionic compound. Web ionic compound definition. The ions have a regular, repeating arrangement called an ionic lattice. Oppositely charged ions of the ionic. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Ionic bonds are atomic bonds created by the. Web the lattice arrangement continues in three dimensions. Covalent crystals are hard, frequently. Briefly discuss three physical properties of ionic solids. These salts commonly exhibit ionic conductivity, which increases with temperature.
Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. Web chemistry chemical bonding and molecular structure formation of ionic compounds ionic compounds what is ionic compound? Web ionic compounds consist of oppositely charged ions that are held together by ionic bonds. Briefly discuss three physical properties of ionic solids. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Web ionic compound definition. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. They can be of a single crystal or powdered form. Covalent crystals are hard, frequently.
The Crystal Lattice Structure of Ionic Compounds infographic diagram
In order to minimize the potential energy of the system, ionic compounds take. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Oppositely charged ions of the ionic. Web ionic compounds form crystal lattices rather than amorphous solids. Web video test 1 2 3 4 the ionic lattice an ionic compound is.
Ionic Compound Properties
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Ionic bonds are atomic bonds created by the. Although molecular compounds form crystals, they frequently take other forms plus. Web crystals are formed when the ionic liquids.
Ionic Bond Definition, Types, Properties & Examples
Covalent crystals are hard, frequently. Web ionic compound definition. The ions have a regular, repeating arrangement called an ionic lattice. Ionic bonds are atomic bonds created by the. Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions.
What Are The Properties Of Ionic Compound slidesharedocs
In order to minimize the potential energy of the system, ionic compounds take. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Briefly discuss three physical properties of ionic solids. They can be of a single crystal or powdered form. Web crystals are formed when the ionic liquids are cooled and get the hard ionic.
Ionic Bond Definition Easy Hard Science
Oppositely charged ions of the ionic. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Ionic bonds are atomic bonds created by the. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. They can be of a single crystal or powdered form.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Ionic bonds are atomic bonds created by the. Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. Sheri neva / getty images. These salts commonly exhibit ionic conductivity, which increases with temperature.
Ionic Bonding Presentation Chemistry
Web ionic compounds form crystal lattices rather than amorphous solids. Sheri neva / getty images. Ionic bonds are atomic bonds created by the. Web ionic compound definition. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under.
What Are The Properties Of Ionic Compound slidesharedocs
Oppositely charged ions of the ionic. Briefly discuss three physical properties of ionic solids. In order to minimize the potential energy of the system, ionic compounds take. They can be of a single crystal or powdered form. Web chemistry chemical bonding and molecular structure formation of ionic compounds ionic compounds what is ionic compound?
Properties of ionic compounds YouTube
Web chemistry chemical bonding and molecular structure formation of ionic compounds ionic compounds what is ionic compound? Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Web ionic compounds form crystal lattices rather than amorphous solids. 11/21/2021 ionic compounds most of the rocks and minerals that surround us are made of ions.
What structural units make up ionic solids? Socratic
Ionic bonds are atomic bonds created by the. These salts commonly exhibit ionic conductivity, which increases with temperature. Covalent crystals are hard, frequently. Ionic crystals consist of alternating cations and anions held together by electrostatic forces. Encyclopedia of physical science and technology (third.
Nacl Or Salt Is An Example Of An Ionic Compound.
Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Covalent crystals are hard, frequently. Web ionic compounds form a crystal lattice. Ionic bonds are atomic bonds created by the.
Web Ionic Compound Definition.
Oppositely charged ions of the ionic. Ionic compounds can be defined as:. These salts commonly exhibit ionic conductivity, which increases with temperature. They can be of a single crystal or powdered form.
Web Ionic Compounds Consist Of Oppositely Charged Ions That Are Held Together By Ionic Bonds.
Web ionic compounds form crystal lattices rather than amorphous solids. The arrangement maximizes the attractive force between oppositely. Briefly discuss three physical properties of ionic solids. Web chemistry chemical bonding and molecular structure formation of ionic compounds ionic compounds what is ionic compound?
11/21/2021 Ionic Compounds Most Of The Rocks And Minerals That Surround Us Are Made Of Ions Held Together Through Ionic Bonding, The Electrical.
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. They can be of a single crystal or powdered form. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions.





.PNG)
.PNG)