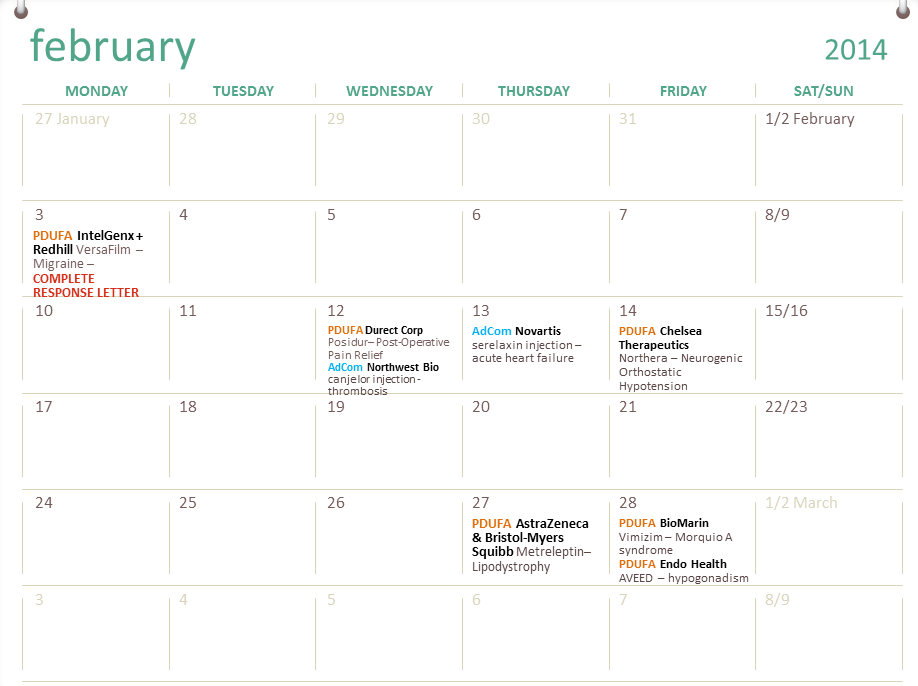
Fda Drug Approval Calendar
Fda Drug Approval Calendar - Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web drug and biologic approval and ind activity reports; Cder drug and biologic approvals for. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web novel drug approvals for 2022 advancing health through innovation: Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Oncology (cancer) / hematologic malignancies. Cder drug and biologic approvals for calendar year 2022.
Oncology (cancer) / hematologic malignancies. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Cder drug and biologic approvals for. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web drug and biologic approval and ind activity reports; Cder drug and biologic approvals for calendar year 2022. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web novel drug approvals for 2022 advancing health through innovation: Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in.
Web novel drug approvals for 2022 advancing health through innovation: Oncology (cancer) / hematologic malignancies. Cder drug and biologic approvals for calendar year 2022. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web drug and biologic approval and ind activity reports; Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Cder drug and biologic approvals for. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web an fda calendar typically displays information about the expected timeline for a particular drug approval.
The Most Important New Drug Of 2014
Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web novel drug approvals for 2022 advancing health through innovation: Web 52 rows below is a listing of new molecular entities and new therapeutic biological products.
FDA Calendar FDA Tracker
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Cder drug and biologic approvals for. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web novel drug approvals for 2022 advancing health through innovation: Web drug and biologic approval and ind activity reports;
FDA Approval Calendar FDA Approval Biotech Stocks
Oncology (cancer) / hematologic malignancies. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web novel drug approvals for 2022 advancing health through innovation: Cder drug and biologic approvals for calendar year 2022. Web below is a listing of new molecular entities and new therapeutic biological products that cder.
Fda Approval Calendar 2023 Everything You Need To Know August 2023
Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web drug and biologic approval and ind activity reports; Cder drug and biologic approvals for. Oncology (cancer) / hematologic malignancies.
FDA Calendar FDA Tracker
Oncology (cancer) / hematologic malignancies. Cder drug and biologic approvals for. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web novel drug approvals for 2022 advancing health through innovation: Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital.
FDA Calendar FDA Tracker
Cder drug and biologic approvals for. Oncology (cancer) / hematologic malignancies. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web novel drug approvals for 2022 advancing health through innovation: Web an fda calendar typically displays information about the expected timeline for a particular drug approval or.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web novel drug approvals for 2022 advancing.
FDA Calendar FDA Tracker
Web drug and biologic approval and ind activity reports; Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web an fda calendar typically displays information about the expected timeline for a.
FDA Calendar FDA Tracker
Web novel drug approvals for 2022 advancing health through innovation: Cder drug and biologic approvals for. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web drug and biologic approval and ind activity reports; Web below is a listing of new molecular entities and new therapeutic biological products that.
Web Drug And Biologic Approval And Ind Activity Reports;
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Cder drug and biologic approvals for.
Cder Drug And Biologic Approvals For Calendar Year 2022.
Oncology (cancer) / hematologic malignancies. Web novel drug approvals for 2022 advancing health through innovation: Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in.