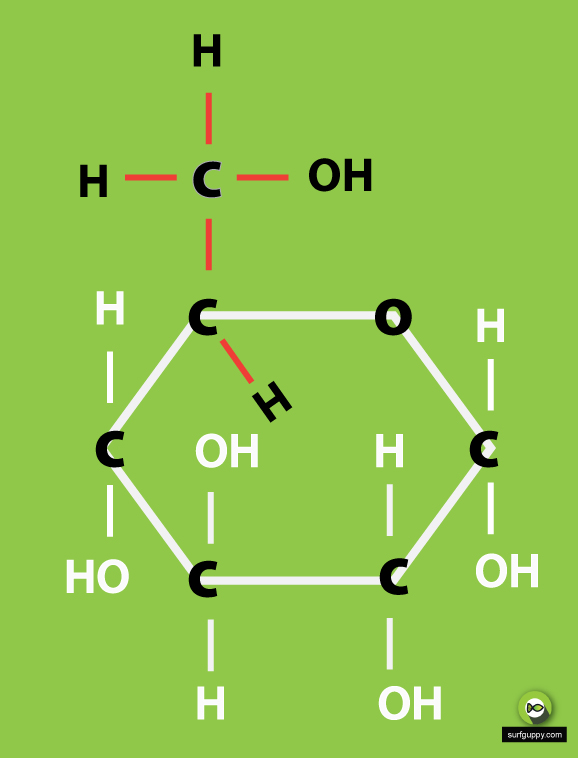
Glucose Ring Form
Glucose Ring Form - Plants and algae prepare glucose during the process of photosynthesis with the help of water, sunlight, and carbon dioxide. Web take a look at the linear form of glucose below. Web glucose molecules form rings. For example, glucose is an aldohexose. When the ring forms, the side chain it closes on is locked into an α or β position. Describe the phenomenon known as mutarotation. Explain, through the use of chemical equations, exactly what happens at the molecular level during the mutarotation process. Web glucose, galactose, and fructose have the same chemical formula ( \text c_6\text h_ {12}\text o_6 c6h12o6 ), but they differ in the organization of their atoms, making them isomers of one another. The atoms in this cyclic molecule then arrange themselves in space to minimize the amount of strain on each of the covalent bonds. Hence, there must have existed an oxide ring between c.
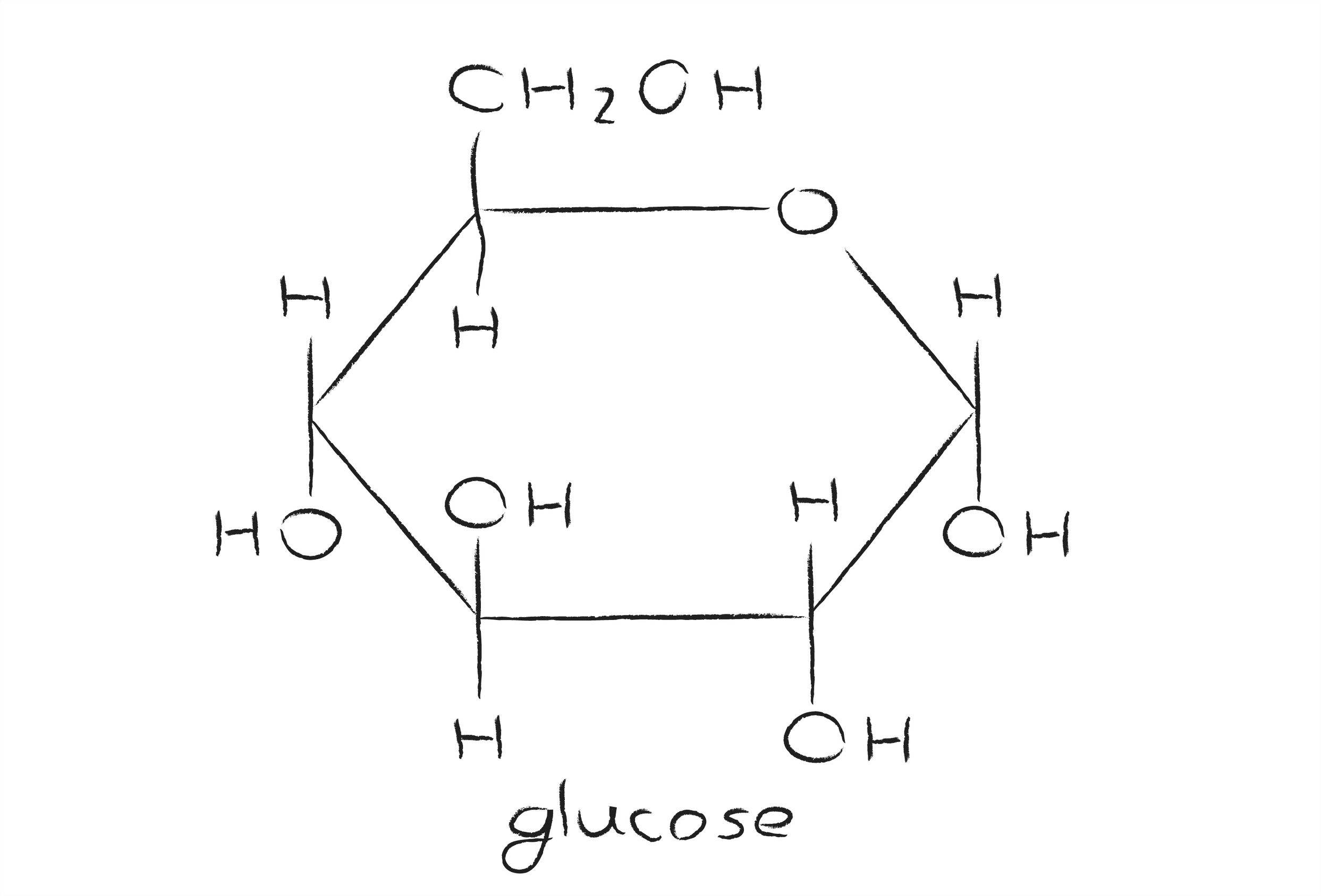
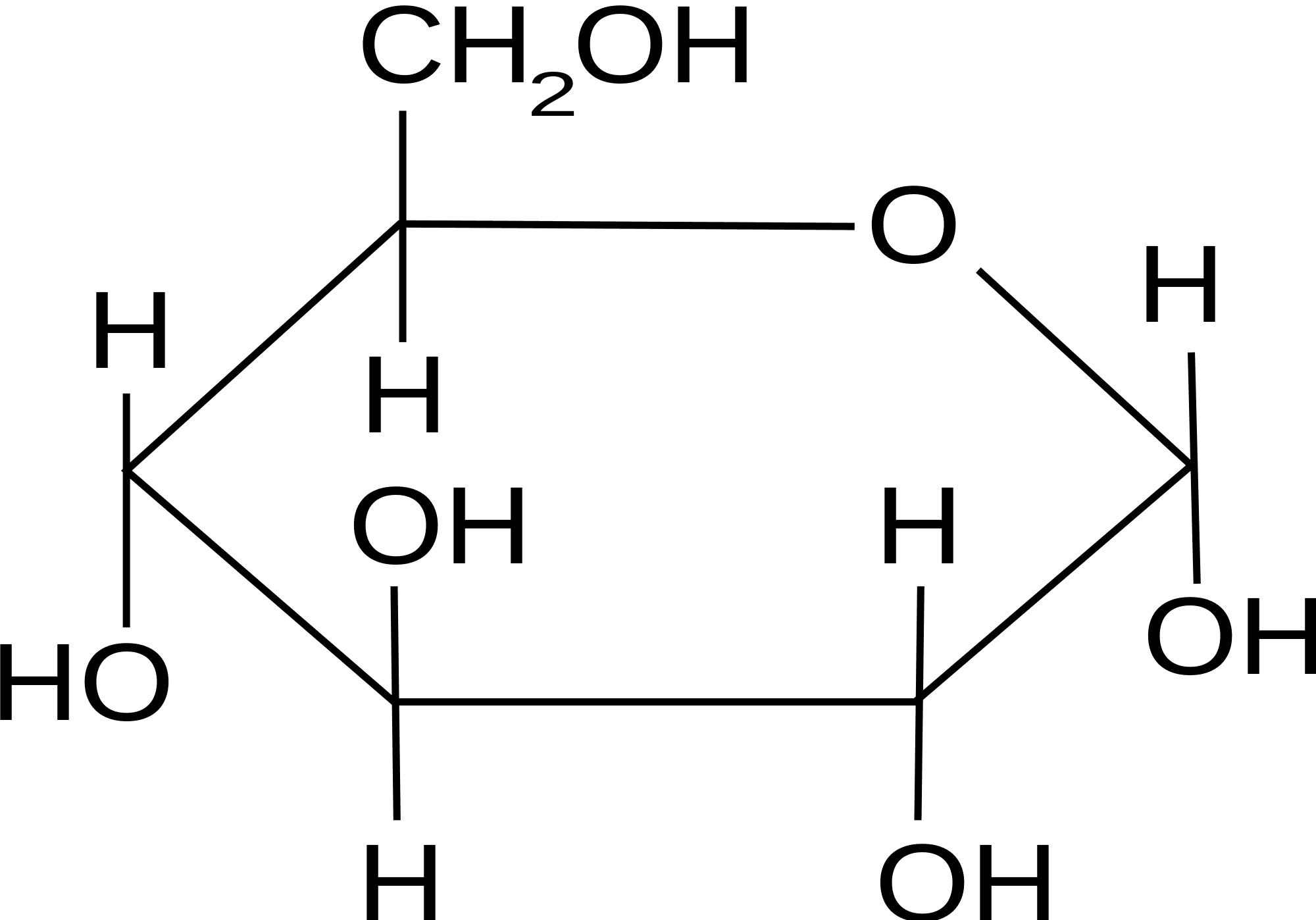
Hence, there must have existed an oxide ring between c. $195.00 (10% off) free shipping. Determine whether a given cyclic pyranose form represents the d or l form of the monosaccharide concerned. Web glucose molecules form rings. This is the same reason that fructose is sweet. Web glucose makes a ring when it is dissolved in an aqueous solution. B, glucose 1 enters sudlow site i and is trapped at the bottom of sudlow site i in pyranose form (left). For example, glucose is an aldohexose. Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. This ring structure of glucose is known as glucopyranose.
B, glucose 1 enters sudlow site i and is trapped at the bottom of sudlow site i in pyranose form (left). This ring structure of glucose is known as glucopyranose. When the ring forms, the side chain it closes on is locked into an α or β position. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. Both rings contain an oxygen atom. Trophozoite ‘rings’ are globose, have a central vacuole, a red chromatin mass and blue cytoplasm; Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis. The primary source of energy required for living organisms is glucose. Five carbon atoms and one oxygen atom, belonging to the aldehydic functional group, make the corners or angles of the hexagon.
Carbohydrate glucose
When the ring forms, the side chain it closes on is locked into an α or β position. The cyclic form of sugars is the favored form in aqueous solution. The ring formed by glucose is hexagonal in structure. Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. Up until.
Biology For Everyone Topic 1 Carbohydrates as energy source and
Web rather, they adopt a cyclic structure (see figure below). Web 1 comment ( 85 votes) upvote flag quantum coding 4 years ago glucose is sweet because it contains oh groups with a certain orientation that interacts with the taste receptor for sweetness in our tongues. The primary source of energy required for living organisms is glucose. Explain, through the.
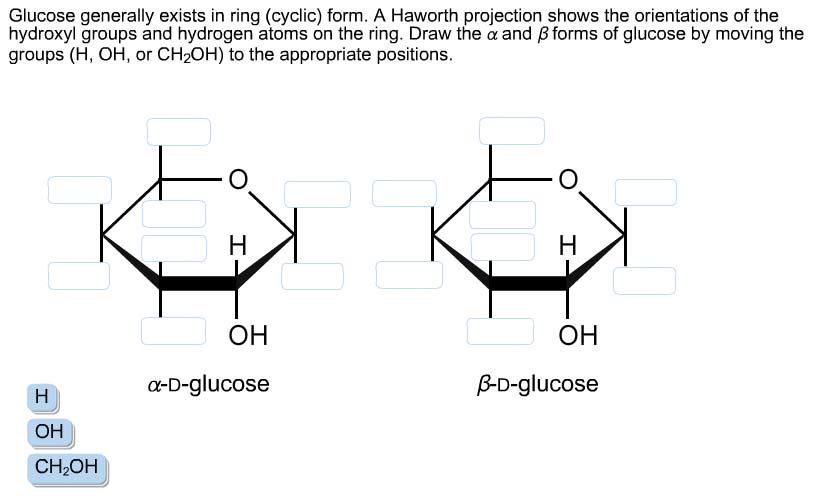
Glucose Generally Exists In Ring (cyclic) Form.
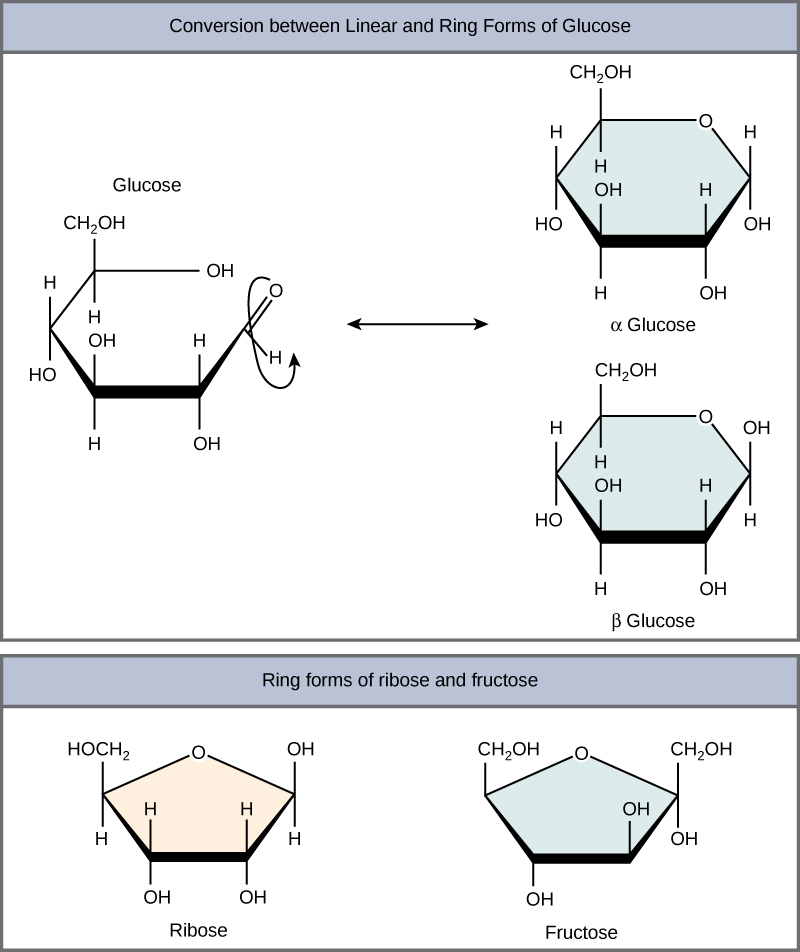
Web glucose, galactose, and fructose have the same chemical formula ( \text c_6\text h_ {12}\text o_6 c6h12o6 ), but they differ in the organization of their atoms, making them isomers of one another. Explain, through the use of chemical equations, exactly what happens at the molecular level during the mutarotation process. Hence, there must have existed an oxide ring between.
Glucose Baking Ingredients BAKERpedia
Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. For example, glucose is an aldohexose. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis. Trophozoite ‘rings’ are globose, have a central vacuole, a red chromatin mass and blue cytoplasm; $195.00 (10% off) free.
The 411 on Dexanhydrous Glucose in Workout Supplements
Trophozoite ‘rings’ are globose, have a central vacuole, a red chromatin mass and blue cytoplasm; For example, glucose is an aldohexose. Web take a look at the linear form of glucose below. Five carbon atoms and one oxygen atom, belonging to the aldehydic functional group, make the corners or angles of the hexagon. Describe the phenomenon known as mutarotation.
Draw the Structure of a Glucose Molecule
Web one of a kind sterling silver brutalist statement ring, artisan sterling branch ring, oxidized sterling free form gemstone ring, studio ring. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. These terms are combined to give full descriptions of individual carbohydrates. Plants and algae prepare glucose during the process of.
16.4 Cyclic Structures of Monosaccharides The Basics of General
Web glucose, galactose, and fructose have the same chemical formula ( \text c_6\text h_ {12}\text o_6 c6h12o6 ), but they differ in the organization of their atoms, making them isomers of one another. Web for glucose in the ring form (pyranose) this is equatorial. Explain, through the use of chemical equations, exactly what happens at the molecular level during the.
Glucose Structure, Properties, Synthesis, Facts & Summary
Web draw, from memory, the cyclic pyranose form of d‑glucose. Web so it makes sense that we're gonna form the most stable ring that we can. This is the same reason that fructose is sweet. With maturation, the ‘rings’ evolve to. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants.
3 Simple Steps Draw the ring structure of glucose molecule
This is the same reason that fructose is sweet. Up until now we have been presenting the structure of glucose as a chain. When the ring forms, the side chain it closes on is locked into an α or β position. Trophozoite ‘rings’ are globose, have a central vacuole, a red chromatin mass and blue cytoplasm; These terms are combined.
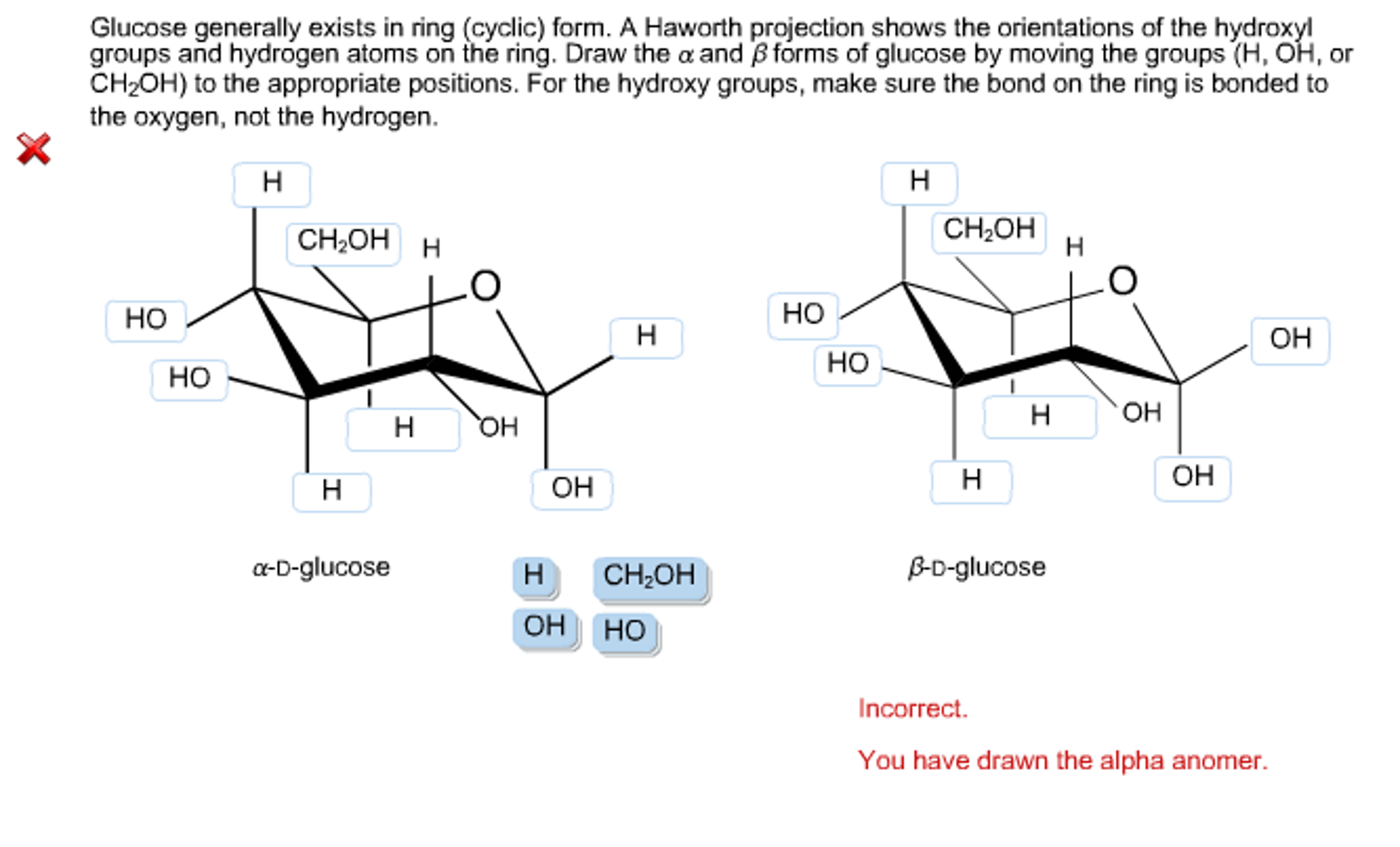
Solved Glucose generally exists in ring (cyclic) form. A
In reality, an aqueous sugar solution contains only 0.02% of the glucose in the chain form, the majority of the structure is in the cyclic chair form. The ring formed by glucose is hexagonal in structure. Web glucose, galactose, and fructose have the same chemical formula ( \text c_6\text h_ {12}\text o_6 c6h12o6 ), but they differ in the organization.
This Is The Same Reason That Fructose Is Sweet.
This ring structure of glucose is known as glucopyranose. These terms are combined to give full descriptions of individual carbohydrates. $195.00 (10% off) free shipping. Web glucose, galactose, and fructose have the same chemical formula ( \text c_6\text h_ {12}\text o_6 c6h12o6 ), but they differ in the organization of their atoms, making them isomers of one another.
Web Rather, They Adopt A Cyclic Structure (See Figure Below).
Explain, through the use of chemical equations, exactly what happens at the molecular level during the mutarotation process. The cyclic form of sugars is the favored form in aqueous solution. B, glucose 1 enters sudlow site i and is trapped at the bottom of sudlow site i in pyranose form (left). Web glucose molecules form rings.
Web Ring Structure For Glucose:
The ring formed by glucose is hexagonal in structure. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis. Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. Determine whether a given cyclic pyranose form represents the d or l form of the monosaccharide concerned.
Web Glucose Makes A Ring When It Is Dissolved In An Aqueous Solution.
With maturation, the ‘rings’ evolve to. This reaction is an example of hemiacetal phase of acetal formation in which an equivalent of alcohol. Describe the phenomenon known as mutarotation. Web 1 comment ( 85 votes) upvote flag quantum coding 4 years ago glucose is sweet because it contains oh groups with a certain orientation that interacts with the taste receptor for sweetness in our tongues.