Study Guide Chapter 6 Section 1 Atoms Elements And Compounds
Study Guide Chapter 6 Section 1 Atoms Elements And Compounds - The study of the building blocks that make up the amazing diversity of life we see today substance a form of matter that has a uniform and unchanging composition i. Each element has a unique name and symbol; A pure substance made of only one kind of atom. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Covalent bond and ionic bond unit 2 chapter 6. The study of matter, its composition, and its properties. Its organized into horizontal rows called. (b) hydrogen is sometimes shown above fluorine on the periodic table?. A compound is a distinct group of atoms. Web main idea of 6.1 matter is composed of tiny particles called atomes what is the science of chemistry?
Web start studying chapter 6 section 1: Web atoms of the same element that have different numbers of neutrons. A compound is a distinct group of atoms. A pure substance made of only one kind of atom. Learn vocabulary, terms, and more with flashcards, games, and other study. Water is composed of hydrogen and oxygen. Web vertical comumns of periodic table of elements. Is a compound in which that atoms are held together by covalent bonds. Web main idea of 6.1 matter is composed of tiny particles called atomes what is the science of chemistry? Web large molecules containing carbon atoms are called micromolecules.
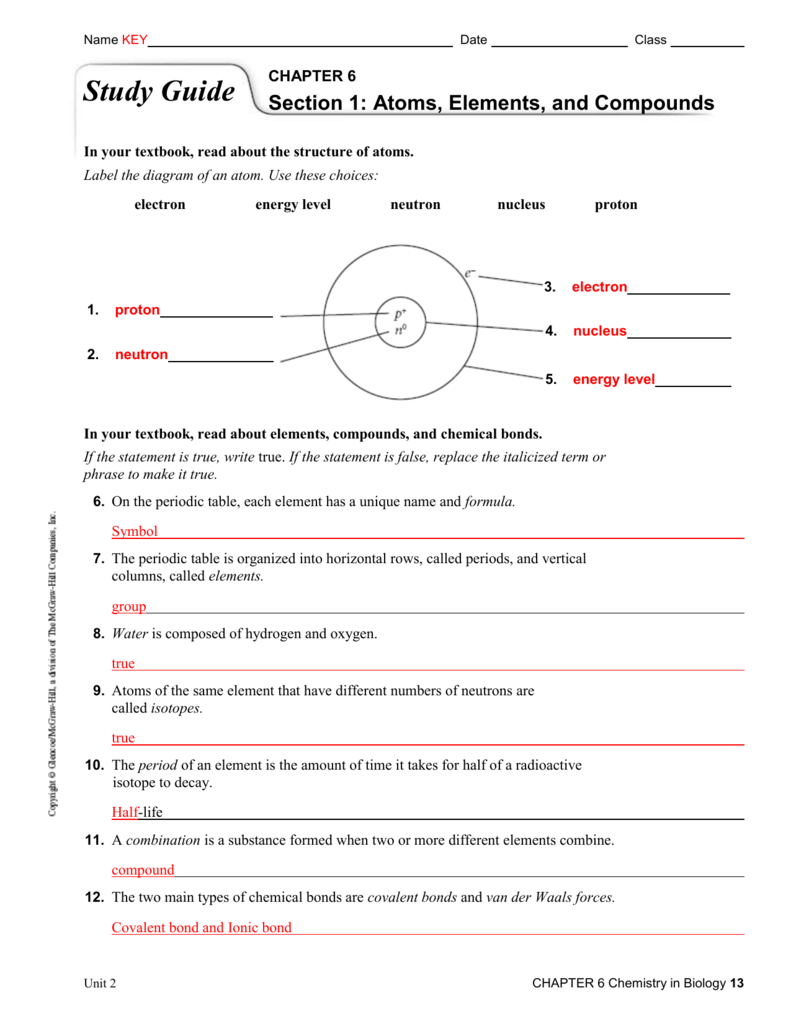
Learn vocabulary, terms, and more with flashcards, games, and other study. Label the diagram of an atom. A pure substance made of only one kind of atom. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Learn with flashcards, games, and more — for free. (a) chemists have put hydrogen and the alkali metals in the same column of the periodic table. Atoms of the same element that have the same number of protons and electrons but have different number of neutrons. Click the card to flip 👆. Web large molecules containing carbon atoms are called micromolecules. The study of matter, its composition, and its properties.
Atoms, elements and compounds academy
Click the card to flip 👆. Web start studying section 6.1 atoms, elements, and compounds. (b) hydrogen is sometimes shown above fluorine on the periodic table?. Web chapter 6 study guide: Its organized into horizontal rows called.
Study guide For chapter 2 Chemistry Atoms, compounds, chemical
Web study guide chapter 6 section 1: A compound in which the atoms. Atomic ratios in compounds chemical formulas for compounds explain the components and quantities of elements in a molecule. Web start studying section 6.1 atoms, elements, and compounds. Web large molecules containing carbon atoms are called micromolecules.
ATOMS ELEMENTS AND COMPOUNDS Poster Lily Keep CalmoMatic
An atom that has given up or gained one or more electrons, carries an electric charge. Atoms of the same element have different. Web start studying section 6.1 atoms, elements, and compounds. Web unit 6 science study guide matter, atoms and elements. Label the diagram of an atom.
Section 3 Atoms, Elements and Compounds
Its organized into horizontal rows called. Web the periodic table is organized into horizontal rows called periods and vertical columns called elements. Atomic ratios in compounds chemical formulas for compounds explain the components and quantities of elements in a molecule. The study of matter, its composition, and its properties. Web chapter 6 section 1:atoms, elements, and compounds in your textbook,.
Chapter 6 study guide key
The two main types of chemical bonds are covalent bonds and van der waals forces. Click the card to flip 👆. The smallest component of an element having the chemical properties of the element. Web chapter 6 study guide: An atom that has given up or gained one or more electrons, carries an electric charge.
Atoms, Elements, and Compound Test Study Guide
Atoms chemistry is the study. Learn vocabulary, terms, and more with flashcards, games, and other study tools. A pure substance made of only one kind of atom. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Atomic ratios in compounds chemical formulas for compounds explain the components and quantities of elements in.
CH3 ATOM, ELEMENTS & COMPOUNDS
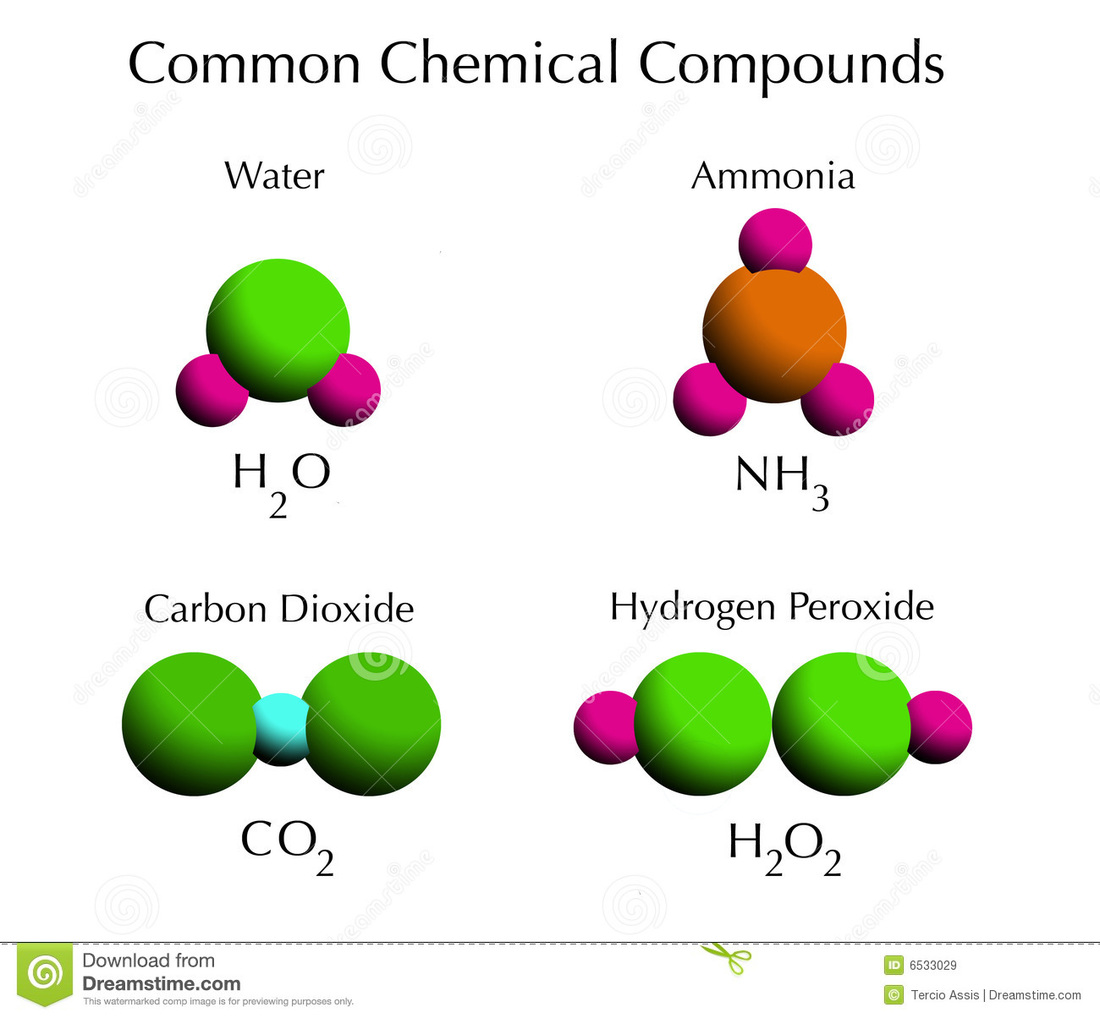
Water is composed of hydrogen and oxygen. The building blocks of life. Web start studying chapter 6 section 1: Electron energy level neutron nucleus proton in your textbook, read about elements, compounds… Atoms of the same element that have the same number of protons and electrons but have different number of neutrons.
Atoms, Elements, & Compounds
The building blocks of matter,. Covalent bond and ionic bond unit 2 chapter 6. (b) hydrogen is sometimes shown above fluorine on the periodic table?. Much of the study of chemistry, however, involves looking at what happens when atoms combine with other atoms to form compounds. A compound is a distinct group of atoms.
Atoms, elements, compounds and mixtures Crossword WordMint
Web study guide chapter 6 section 1: Discover the properties of atomic ratios, their relationship to. Web bio study guide chapter 6 section 1: Much of the study of chemistry, however, involves looking at what happens when atoms combine with other atoms to form compounds. A pure substance made of only one kind of atom.
Chemistry In Biology Chapter 6 Section 4 Study Guide Answers Study Poster
Web large molecules containing carbon atoms are called micromolecules. A pure substance formed when two or more different elements combine. The smallest component of an element having the chemical properties of the element. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. • covalent compounds can be solids, liquids, or gases.
A Pure Substance Made Of Only One Kind Of Atom.
Atomic ratios in compounds chemical formulas for compounds explain the components and quantities of elements in a molecule. Covalent bond and ionic bond unit 2 chapter 6. Web atoms of the same element that have different numbers of neutrons. Label the diagram of an atom.
• Covalent Compounds Can Be Solids, Liquids, Or Gases.
Anything that has mass and takes up space, made up of tiny individual particles (atoms) atoms. An atom that has given up or gained one or more electrons, carries an electric charge. Water is composed of hydrogen and oxygen. The smallest component of an element having the chemical properties of the element.
Its Organized Into Horizontal Rows Called.
Web study guide chapter 6 section 1: The study of matter, its composition, and its properties. (b) hydrogen is sometimes shown above fluorine on the periodic table?. Web matter is composed of tiny particles called atoms.
Web The Periodic Table Is Organized Into Horizontal Rows Called Periods And Vertical Columns Called Elements.
Web main idea of 6.1 matter is composed of tiny particles called atomes what is the science of chemistry? A pure substance formed when two or more different elements combine. Much of the study of chemistry, however, involves looking at what happens when atoms combine with other atoms to form compounds. The study of the building blocks that make up the amazing diversity of life we see today substance a form of matter that has a uniform and unchanging composition i.