When Ice Melts To Form Liquid Energy Is
When Ice Melts To Form Liquid Energy Is - Holding 100ml of water (ebkare)________________2. Web as soon as the temperature increases and is above 0°c, the ice begins to melt. Thermal energy is transferred to ice causing this to occur. Web for example, ice is water in the solid state: Eventually, the components of the liquid will reach thermal equilibrium, as predicted by the second law of thermodynamics—that. Web by liz veloz. Web the ice cubes are at the melting temperature of 0 °c °c. At what temperature does water freeze? Water in the liquid state becomes ice when it is cooled down Web ask students to investigate whether ice exposed to warm or room temperature air would melt more quickly or more slowly than ice exposed to still or flowing warm or room.
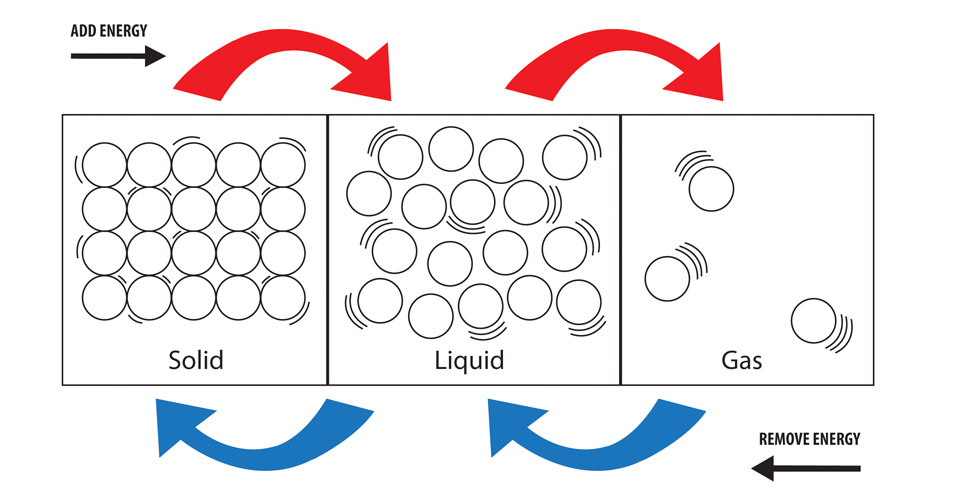
Web for example, ice is water in the solid state: H 2 o, water, or dihydrogen monoxide, is a molecule composed of one oxygen atom and two hydrogen atoms. Water in the liquid state becomes ice when it is cooled down Energy is released when the water condenses, speeding the melting of the. If the supplied energy is even larger, then atoms completely move away from each other turning liquid. First, the phase change occurs. Web melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. If there is enough energy (heat) available to the ice, it absorbs that and changes its phase from. Eventually, the components of the liquid will reach thermal equilibrium, as predicted by the second law of thermodynamics—that. Heat is transferred from the soda to the ice for melting.
In other words, ice melts. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Water in the liquid state becomes ice when it is cooled down Web in the case of water melting from ice into water, it is transitioning from a solid to liquid. Ice melts faster in water than in soda. At what temperature does water freeze? Web as soon as the temperature increases and is above 0°c, the ice begins to melt. Web ice melts at 0 °c to give water at 0 °c. Eventually, the components of the liquid will reach thermal equilibrium, as predicted by the second law of thermodynamics—that. This occurs when the internal energy of the solid increases,.
Chapitre 1 Section A Some Basic Definitions
Water in the liquid state becomes ice when it is cooled down In other words, ice melts. Ice melts to form water in the liquid state when it is heated; That means energy is being added in the form of heat energy. Heat is transferred from the soda to the ice for melting.
Liquid Ice Melt Non Chloride Ice Melt
Web melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This is because soda has sodium (salt) in it, and adding sodium makes ice melt more slowly than it will in plain. H 2 o, water, or dihydrogen monoxide, is a molecule composed of one oxygen atom.
Melting Ice= Solid to Liquid Apples and ABC's
Web by liz veloz. Web ask students to investigate whether ice exposed to warm or room temperature air would melt more quickly or more slowly than ice exposed to still or flowing warm or room. Web in the case of water melting from ice into water, it is transitioning from a solid to liquid. If the supplied energy is even.
Liquid Ice Melt Melts Away (5 Gallon) Liquid Ice Melt Liquid Ice M
Energy is released when the water condenses, speeding the melting of the. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Web by liz veloz. This occurs when the internal energy of the solid increases,. Ice melts faster in water than in soda.
Drinkable Review Liquid Ice
Thermal energy is transferred to ice causing this to occur. If there is enough energy (heat) available to the ice, it absorbs that and changes its phase from. That means energy is being added in the form of heat energy. At what temperature does water freeze? Web in the case of water melting from ice into water, it is transitioning.
Liquid Energy Ice available in patriotic packaging 20170714
This occurs when the internal energy of the solid increases,. Ice melts faster in water than in soda. Water in the liquid state becomes ice when it is cooled down Web figure 12.9 the ice in this drink is slowly melting. Heat is transferred from the soda to the ice for melting.
icemelts Rasevic Companies
Thermal energy is transferred to ice causing this to occur. Web ice melts at 0 °c to give water at 0 °c. Web as soon as the temperature increases and is above 0°c, the ice begins to melt. Heat is transferred from the soda to the ice for melting. Web for example, ice is water in the solid state:
The Ice Is Melting Learn Something New
This occurs when the internal energy of the solid increases,. Web by liz veloz. These atom are held together by a covalent. Web ice is water in a solid form and when it melts it turns into a liquid, which is actually a transition into a different phase, or what a lot of scientists call a “phase transformation”. Ice melts.
What happens to the energy of its molecules as ice melts into
Web figure 12.9 the ice in this drink is slowly melting. Web as soon as the temperature increases and is above 0°c, the ice begins to melt. Water in the liquid state becomes ice when it is cooled down At what temperature does water freeze? Water in the liquid state becomes ice when it is cooled down
What Occurs When Matter Transitions Between a Solid, Liquid & Gas
At what temperature does water freeze? Water in the liquid state becomes ice when it is cooled down This is because soda has sodium (salt) in it, and adding sodium makes ice melt more slowly than it will in plain. Melting of ice occurs in two steps: H 2 o, water, or dihydrogen monoxide, is a molecule composed of one.
That Means Energy Is Being Added In The Form Of Heat Energy.
Web figure 12.9 the ice in this drink is slowly melting. This is because soda has sodium (salt) in it, and adding sodium makes ice melt more slowly than it will in plain. First, the phase change occurs. Web for example, ice is water in the solid state:
Web By Liz Veloz.
Ice melts faster in water than in soda. Web for example, ice is water in the solid state: Web in the case of water melting from ice into water, it is transitioning from a solid to liquid. Web activity introduce the activity by discussing the freezing temperatures of water and the melting temperatures of ice.
This Occurs When The Internal Energy Of The Solid Increases,.
Eventually, the components of the liquid will reach thermal equilibrium, as predicted by the second law of thermodynamics—that. H 2 o, water, or dihydrogen monoxide, is a molecule composed of one oxygen atom and two hydrogen atoms. Melting of ice occurs in two steps: Web the ice cubes are at the melting temperature of 0 °c °c.
Thermal Energy Is Transferred To Ice Causing This To Occur.
Web ice is water in a solid form and when it melts it turns into a liquid, which is actually a transition into a different phase, or what a lot of scientists call a “phase transformation”. Energy is released when the water condenses, speeding the melting of the. If there is enough energy (heat) available to the ice, it absorbs that and changes its phase from. Web ask students to investigate whether ice exposed to warm or room temperature air would melt more quickly or more slowly than ice exposed to still or flowing warm or room.