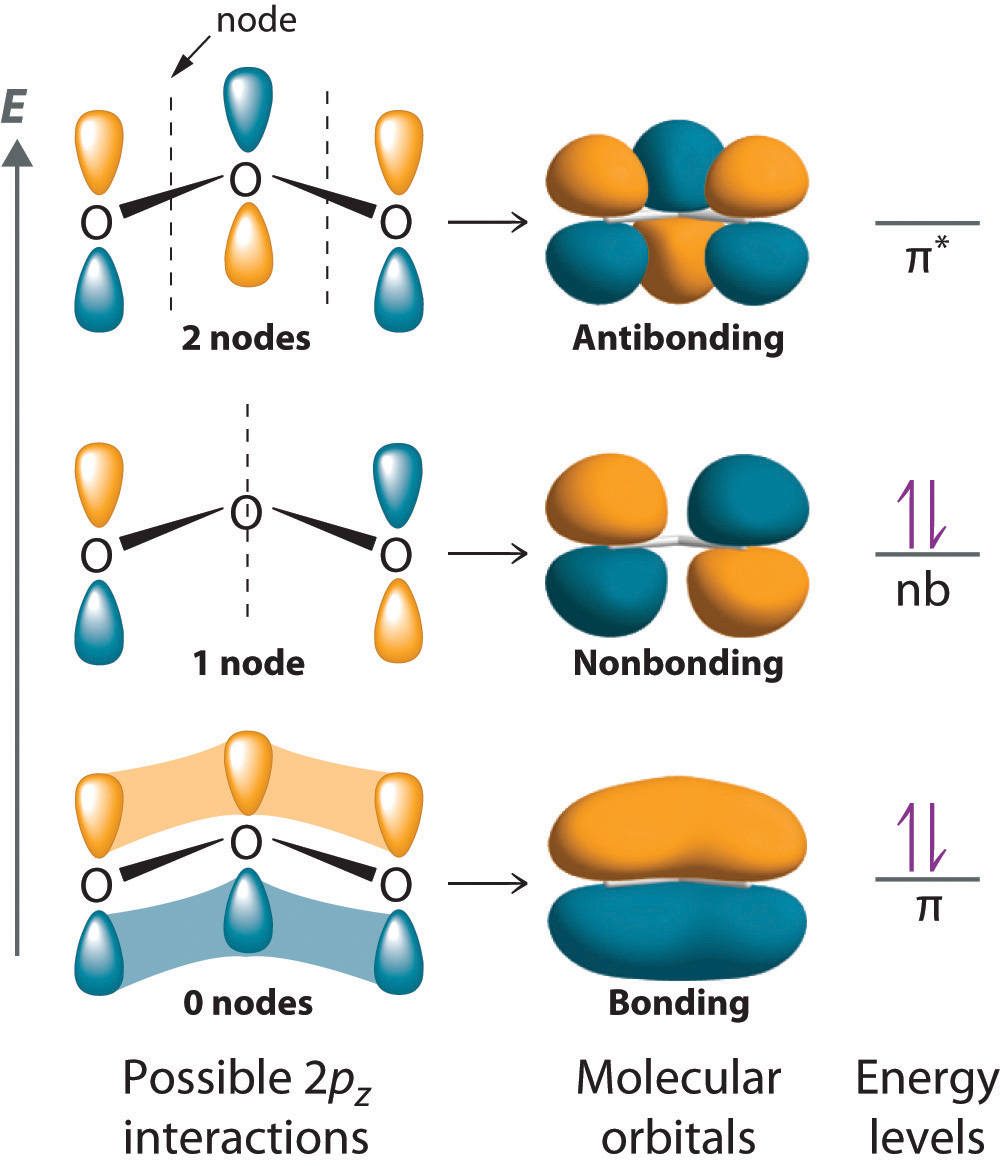
Which Bonds Form In The Reaction Shown In The Diagram
Which Bonds Form In The Reaction Shown In The Diagram - Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web there are many types of chemical bonds and forces that bind molecules together. Illustrate covalent bond formation with lewis electron dot diagrams. Types of chemical bonds including. In endothermic reactions, the reactants have. One single bond between atoms and three lone pairs of electrons per atom. Web we have in this case, the reaction; The structure and bonding in a substance are modelled in different ways, including dot and. The two most basic types of bonds are characterized as either ionic or. Web which bonds form in the reaction shown in the diagram?
So yes, we can have hydrogen bonding between one h2o. Web in an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. In other words, the energy of the products is. A look at ionic bonding, where positive and negative ions attract each other and combine. The two most basic types of bonds are characterized as either ionic or. One single bond between atoms and three lone pairs of electrons per atom. Web the other halogen molecules (f 2, br 2, i 2, and at 2) form bonds like those in the chlorine molecule: It occurs between molecules when hydrogen is covalently bound to a strongly electronegative. Co2 has two double bonds between the carbon atom and each of the two. Whether a reaction is endothermic or exothermic depends on the difference between the.
So yes, we can have hydrogen bonding between one h2o. Web chemical bonds google classroom chemical bonds hold molecules together and create temporary connections that are essential to life. Web the oppositely charged ions are strongly attracted to each other, forming ionic bonds. It occurs between molecules when hydrogen is covalently bound to a strongly electronegative. Web energy is released when new bonds form. Web there are many types of chemical bonds and forces that bind molecules together. As additional monomers join via multiple. Web which bonds form in the reaction shown in the diagram? In endothermic reactions, the reactants have. The two most basic types of bonds are characterized as either ionic or.
Construction Bonds LP Compariqo
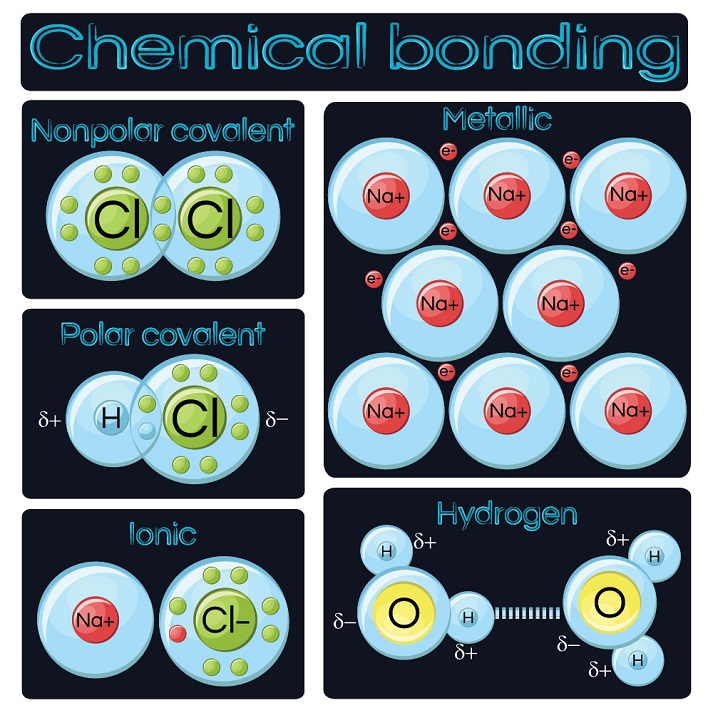
Whether a reaction is endothermic or exothermic depends on the difference between the. Web such bonds are called covalent bonds. Ionic bonding typically occurs when it is easy. In this case, we have the following bonds being formed in the process of the reaction; Web ionic bonds, covalent bonds and metallic bonds are examples of chemical bonds.
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
Web the monomers that are joined via dehydration synthesis reactions share electrons and form covalent bonds with each other. The bonds between the two hydrogen atoms and. Web hydrogen bonding is an intermolecular interaction, i.e. In other words, the energy of the products is. The structure and bonding in a substance are modelled in different ways, including dot and.
PPT Chapter 7. Chemical Bonds PowerPoint Presentation, free download
It occurs between molecules when hydrogen is covalently bound to a strongly electronegative. Web there are many types of chemical bonds and forces that bind molecules together. Whether a reaction is endothermic or exothermic depends on the difference between the. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both..
Question Video Recalling the Type of Bond That Forms between
Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web in an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. The bonds between the two hydrogen atoms and. Illustrate covalent bond formation with lewis electron dot diagrams. Web.
Walkthrough of Substitution Reactions (1) Introduction
The bonds between the two hydrogen atoms and. Web covalent bonds and molecules a covalent bond is formed when two atoms share electron pairs. Web which bonds form in the reaction shown in the diagram? So, there is 1 o=o bond in o2. Web the oppositely charged ions are strongly attracted to each other, forming ionic bonds.
2.2A Covalent Bonds and Other Bonds and Interactions Medicine LibreTexts
Web there are many types of chemical bonds and forces that bind molecules together. One single bond between atoms and three lone pairs of electrons per atom. The structure and bonding in a substance are modelled in different ways, including dot and. Illustrate covalent bond formation with lewis electron dot diagrams. So yes, we can have hydrogen bonding between one.
Solved Which statement best describes why covalent bonds
In this case, we have the following bonds being formed in the process of the reaction; The structure and bonding in a substance are modelled in different ways, including dot and. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Illustrate covalent bond formation with lewis.
Bond Order Definition in Chemistry
Web in an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. Ionic bonding typically occurs when it is easy. Web hydrogen bonding is an intermolecular interaction, i.e. The two most basic types of bonds are characterized as either ionic or. A look at ionic bonding, where positive and negative ions attract each.
11.6 Delocalized Electrons Bonding in the Benzene Molecule
Web in an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. In this case, we have the following bonds being formed in the process of the reaction; Web such bonds are called covalent bonds. Web there are many types of chemical bonds and forces that bind molecules together. Web the type of.
Metallic Bonding Meaning, Properties, Factors Embibe
The two most basic types of bonds are characterized as either ionic or. Web there are many types of chemical bonds and forces that bind molecules together. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web covalent bonds and molecules a covalent bond is formed.
Web We Have In This Case, The Reaction;
Web in an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. Web the other halogen molecules (f 2, br 2, i 2, and at 2) form bonds like those in the chlorine molecule: In a covalent bond, the stability of the bond comes from the shared electrostatic. So yes, we can have hydrogen bonding between one h2o.
Whether A Reaction Is Endothermic Or Exothermic Depends On The Difference Between The.
One single bond between atoms and three lone pairs of electrons per atom. Web energy is released when new bonds form. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both. Web which bonds form in the reaction shown in the diagram?
Web Covalent Bonds And Molecules A Covalent Bond Is Formed When Two Atoms Share Electron Pairs.
O2 has a double bond between the two oxygen atoms. The structure and bonding in a substance are modelled in different ways, including dot and. Types of chemical bonds including. In other words, the energy of the products is.
Web Hydrogen Bonds Can Form Between Different Molecules, As Long As One Molecule Has H And The Other Has N, O, Or F.
It occurs between molecules when hydrogen is covalently bound to a strongly electronegative. A look at ionic bonding, where positive and negative ions attract each other and combine. Web the monomers that are joined via dehydration synthesis reactions share electrons and form covalent bonds with each other. Illustrate covalent bond formation with lewis electron dot diagrams.

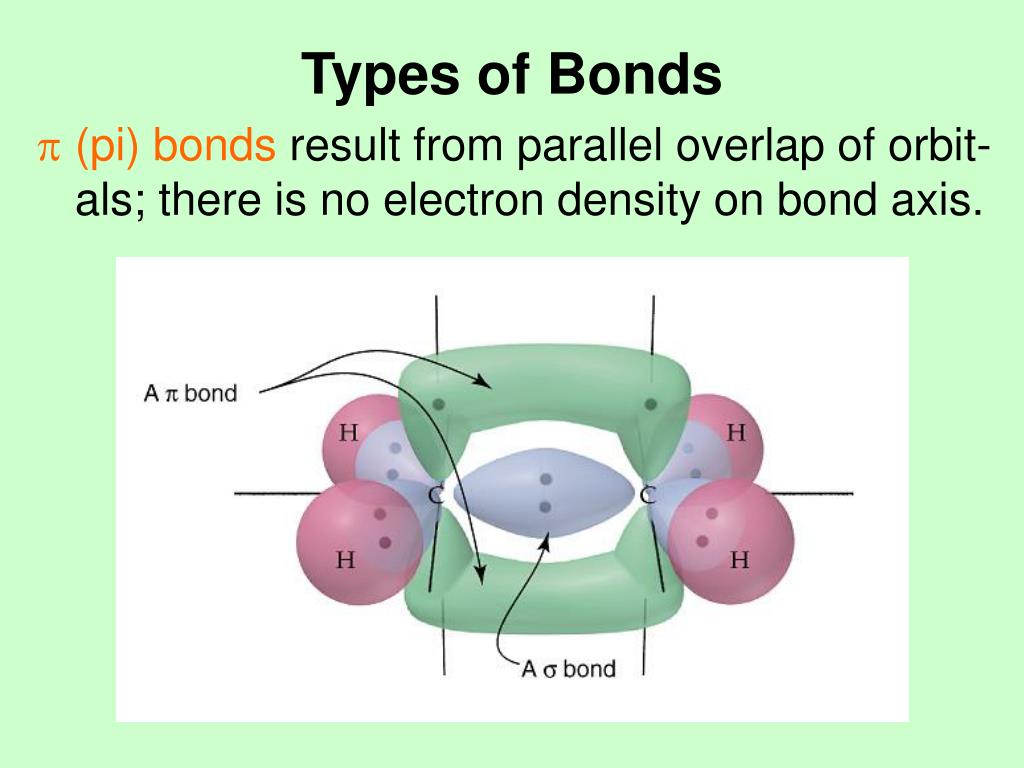



/molecular-model-illustration-545862141-5782ca213df78c1e1f55b395.jpg)