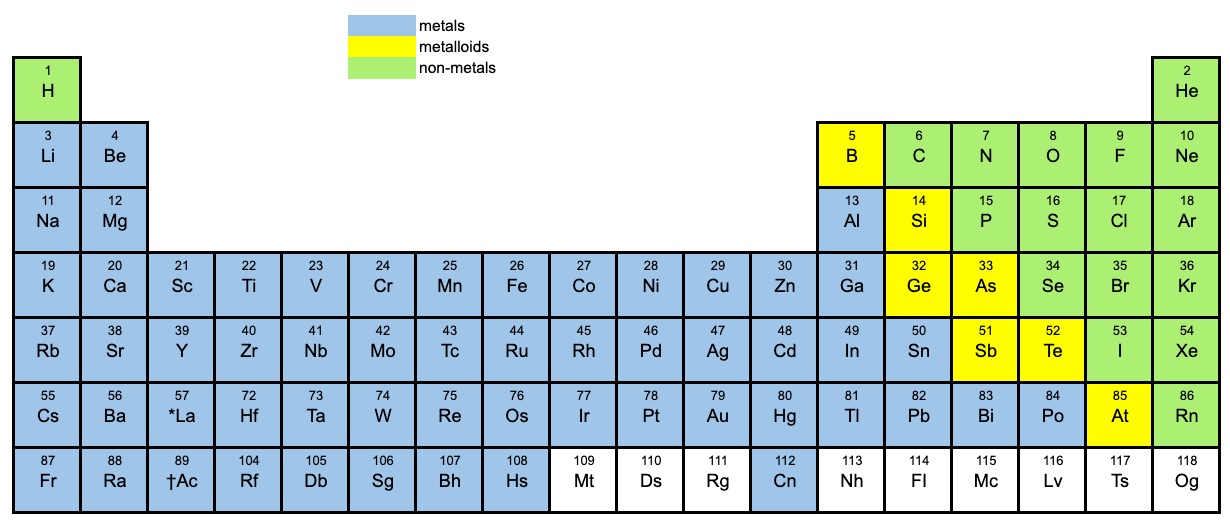
Which Elements Can Form Acidic Compounds
Which Elements Can Form Acidic Compounds - A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the. Web some compounds contain polyatomic ions; Sodium and potassium have low melting points. Sodium and magnesium oxides are alkaline. Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic acid. Web the following elements that can form acidic compounds are the following: These solutions are named by adding the. Web the elements that can form acidic compounds are; Hc 2 h 3 o 2 also known as: Chemical properties of metals metals are.
Web acidic oxides will typically have a low pka and may be inorganic or organic. The names of common polyatomic ions should be memorized. Web laguna design / getty images acetic acid: Web the following elements that can form acidic compounds are the following: Web the oxides of the top of group 4 elements are slightly acidic, and the acidity of the oxides decreases down the group. For much of organic chemistry, an acid may be defined as a compound that can transfer a proton (h +) to a base, and a base may be defined as any. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than. Aluminium oxides are amphoteric (reacting both as a base or acid). Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic acid. These solutions are named by adding the.
Web an oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Hc 2 h 3 o 2 also known as: Web the elements that can form acidic compounds are; Acetic acid is found in vinegar. Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic acid. Silicon, phosphorus, sulfur, and chlorine oxides. Sulfur, arsenic, selenium, antimony, silicon. Aluminium oxides are amphoteric (reacting both as a base or acid). Web laguna design / getty images acetic acid: Alkalis dissolve in water to give a ph greater than 7.
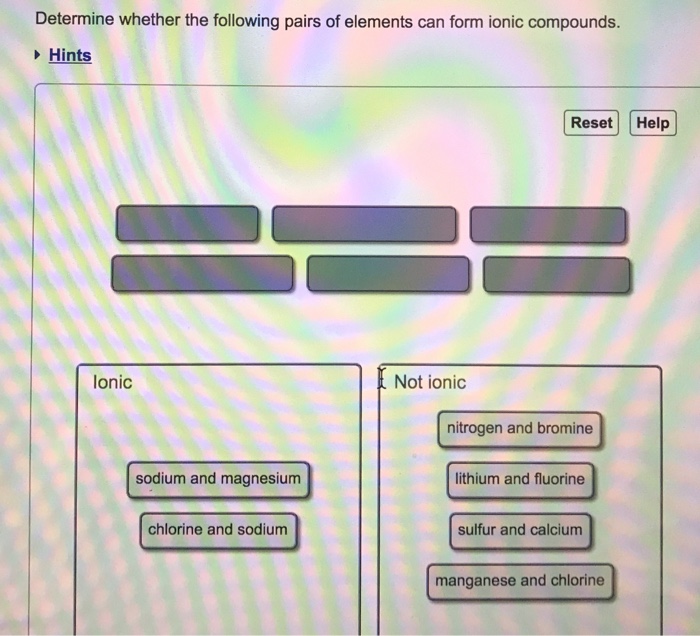
Solved Determine Whether The Following Pairs Of Elements
Sulfur, arsenic, selenium, antimony, silicon. The names of common polyatomic ions should be memorized. Acidic compounds are formed by nonmetals. Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic acid. Hc 2 h 3 o 2 also known as:
Chemistry Writing/Naming Acidic Compounds YouTube
A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the. Web which elements can form acidic compounds? A ph less than 7 is acidic. These solutions are named by adding the. Simple covalent compounds that contain hydrogen, such as hcl, hbr, and hcn, often dissolve in water to produce acids.
Acids — Definition & Overview Expii
Simple covalent compounds that contain hydrogen, such as hcl, hbr, and hcn, often dissolve in water to produce acids. Web tungsten has the highest melting point where as silver has low boiling point. Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic.
Solved 1. Rank the following compounds from least acidic to
Acidic compounds are formed by nonmetals. Silicon, phosphorus, sulfur, and chlorine oxides. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than. Alkalis dissolve in water to give a ph greater than 7.
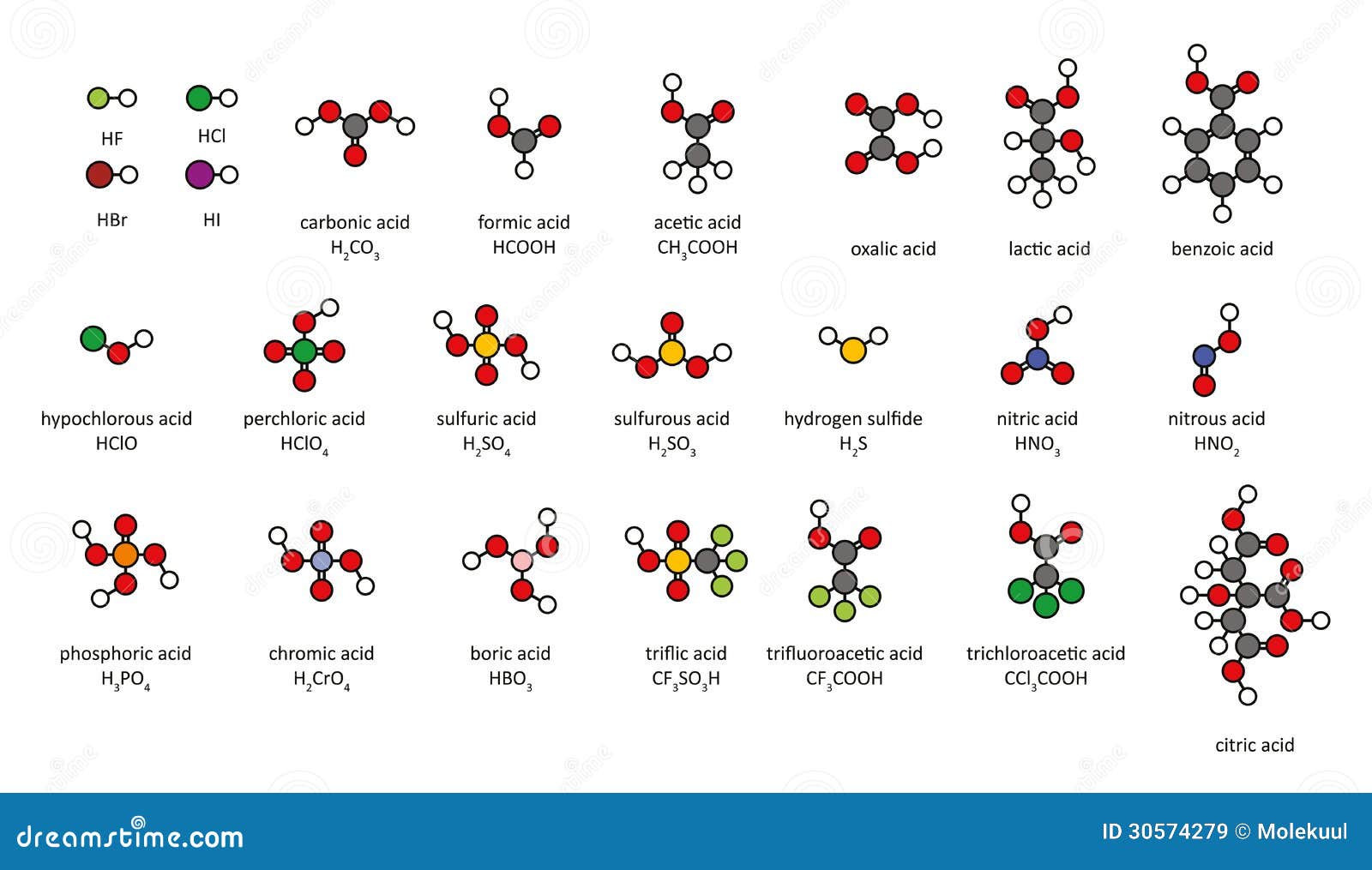
Common Acids, 2D Chemical Structures. Royalty Free Stock Images Image
More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than. Web the elements that can form acidic compounds are; Web because fluoride is the least stable (most basic) of the halide conjugate bases, hf is the least acidic of the haloacids, only slightly stronger than acetic acid. Copper can form ions.
pešiak nesmierne nákup boric acid molecular weight 10 svätyne detektor
Silicon, phosphorus, sulfur, and chlorine oxides. Aluminium oxides are amphoteric (reacting both as a base or acid). Chemical properties of metals metals are. Web which elements can form acidic compounds? Web the oxides of the top of group 4 elements are slightly acidic, and the acidity of the oxides decreases down the group.
The Chemistry of Ion Exchange WCP Online
Web tungsten has the highest melting point where as silver has low boiling point. Sulfur rubidium arsenic selenium silicon xenon antimony Sodium and potassium have low melting points. For much of organic chemistry, an acid may be defined as a compound that can transfer a proton (h +) to a base, and a base may be defined as any. Web.
1. Naming compounds High School/Honors/AP® Chemistry
Chemical properties of metals metals are. A ph equal to 7. Sodium and magnesium oxides are alkaline. Web the oxides of the top of group 4 elements are slightly acidic, and the acidity of the oxides decreases down the group. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the.
PPT Elements & Compounds PowerPoint Presentation, free download ID
The ph scale measures the acidity or alkalinity of a solution. Simple covalent compounds that contain hydrogen, such as hcl, hbr, and hcn, often dissolve in water to produce acids. Sodium and potassium have low melting points. Web the oxides of the top of group 4 elements are slightly acidic, and the acidity of the oxides decreases down the group..
AcidBase & Solvents The Chemistry Guru
Acidic compounds are formed by nonmetals. Alkalis dissolve in water to give a ph greater than 7. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the. Acetic acid is found in vinegar. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than.
Copper Can Form Ions With A 1+ Or 2+ Charge, And Iron Can Form Ions With A 2+ Or 3+ Charge.
Sodium and potassium have low melting points. Sodium and magnesium oxides are alkaline. Web tungsten has the highest melting point where as silver has low boiling point. Aluminium oxides are amphoteric (reacting both as a base or acid).
Hc 2 H 3 O 2 Also Known As:
Web some compounds contain polyatomic ions; Sulfur, arsenic, selenium, antimony, silicon. Silicon, phosphorus, sulfur, and chlorine oxides. Chemical properties of metals metals are.
Sulfur Rubidium Arsenic Selenium Silicon Xenon Antimony
Molecular compounds can form compounds. For much of organic chemistry, an acid may be defined as a compound that can transfer a proton (h +) to a base, and a base may be defined as any. Simple covalent compounds that contain hydrogen, such as hcl, hbr, and hcn, often dissolve in water to produce acids. Acetic acid is found in vinegar.
Web The Oxides Of The Top Of Group 4 Elements Are Slightly Acidic, And The Acidity Of The Oxides Decreases Down The Group.
These solutions are named by adding the. Acidic compounds are formed by nonmetals. Alkalis dissolve in water to give a ph greater than 7. The names of common polyatomic ions should be memorized.


:max_bytes(150000):strip_icc()/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png)