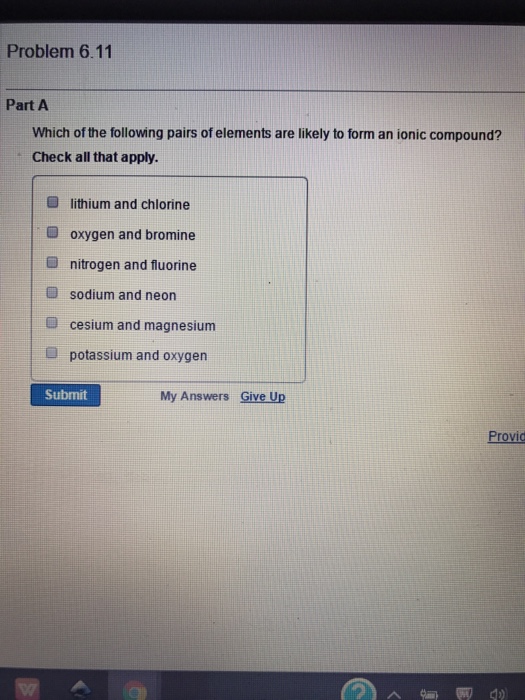
Which Of These Pairs Would Form An Ionic Bond
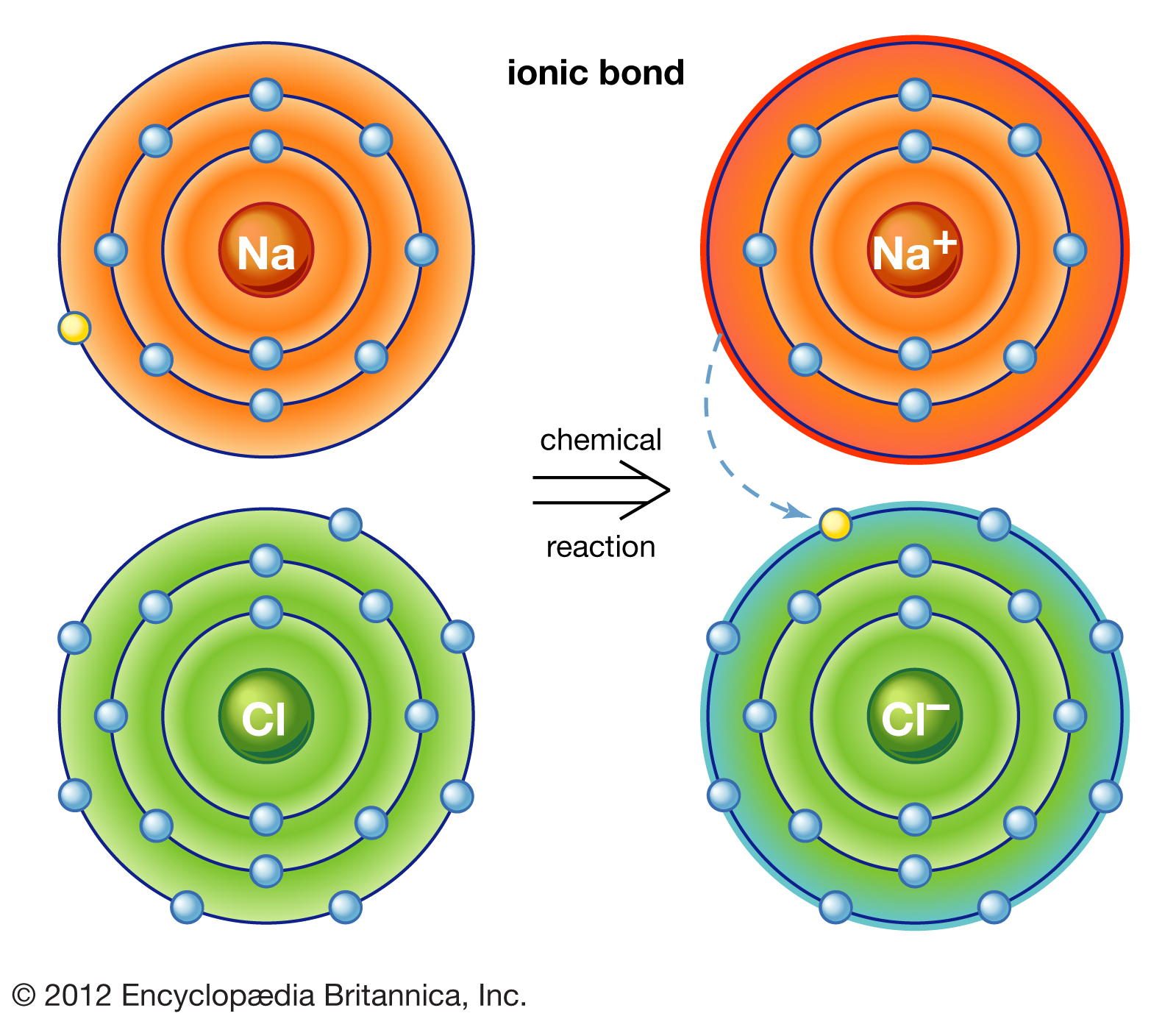
Which Of These Pairs Would Form An Ionic Bond - The attraction of oppositely charged ions caused by electron. Predict whether each pair would form ionic. So just choose the one with a nonmetal element (like na) and a metal element (like cl) yes, k and br. Ionic bonds form between nonmetals and metals. These oppositely charged ions attract each other to form ionic networks (or lattices. Web br (bromine) belongs to viia, and has _____ 7 valence electrons, explain the process of ionic bond formation between k (potassium, a metal) and br (bromine, a nonmetal). Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. These oppositely charged ions attract each other to form ionic networks (or lattices). Look at each of the following pairs of elements. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms.
These oppositely charged ions attract each other to form ionic networks (or lattices. Bonding between a metal and a nonmetal is often ionic. So just choose the one with a nonmetal element (like na) and a metal element (like cl) yes, k and br. Some compounds contain both covalent and ionic bonds. Web the tendency to form species that have eight electrons in the valence shell is called the octet rule. Ionic bonds form between nonmetals and metals. These oppositely charged ions attract each other to form ionic networks (or lattices). Web bonds between two nonmetals are generally covalent; Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web which of these pairs would form an ionic bond?
Web br (bromine) belongs to viia, and has _____ 7 valence electrons, explain the process of ionic bond formation between k (potassium, a metal) and br (bromine, a nonmetal). Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web which of these pairs would form an ionic bond? Bonding between a metal and a nonmetal is often ionic. Web answered which of these pairs would form an ionic bond? Look at each of the following pairs of elements. These oppositely charged ions attract each other to form ionic networks (or lattices). Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. These oppositely charged ions attract each other to form ionic networks (or lattices. I got it right duhhh choose all the.
Ionic Bond Definition, Types, Properties & Examples
These oppositely charged ions attract each other to form ionic networks (or lattices. Bonding between a metal and a nonmetal is often ionic. Web the tendency to form species that have eight electrons in the valence shell is called the octet rule. Ionic bonds form between nonmetals and metals. Web br (bromine) belongs to viia, and has _____ 7 valence.
Ionic Bond Definition, Types, Properties & Examples
Predict whether each pair would form ionic. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. These oppositely charged ions attract each other to form ionic.
Solved Which of the following pairs of elements are likely
Bonding between a metal and a nonmetal is often ionic. K and br answers answer 1 is there any other pairs answer 2 answer: They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web the tendency to form species that have eight electrons in the valence shell is called the octet rule. I got.
Solved Which Of These Pairs Of Elements Would Be Most Lik...
These oppositely charged ions attract each other to form ionic networks (or lattices. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web the tendency to form species that have eight electrons in the valence shell.
Which Of These Pairs Of Elements Would Be Most Lik...
Web br (bromine) belongs to viia, and has _____ 7 valence electrons, explain the process of ionic bond formation between k (potassium, a metal) and br (bromine, a nonmetal). Look at each of the following pairs of elements. Electron transfer produces negative ions called anions and. These oppositely charged ions attract each other to form ionic networks (or lattices). Predict.
Ionic Bond Definition, Types, Properties & Examples
They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Ionic bonds form between nonmetals and metals. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Bonding between a metal and a nonmetal is often ionic. Web br (bromine) belongs to viia, and has _____ 7.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Ionic bonds form between nonmetals and metals. Predict whether each pair would form ionic. K and br answers answer 1 is there any other pairs answer 2 answer: Electron transfer produces negative ions called anions and.
Solved Which of the following pairs of elements are likely
Predict whether each pair would form ionic. These oppositely charged ions attract each other to form ionic networks (or lattices). Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web br (bromine) belongs to viia, and has _____ 7 valence electrons, explain the process of ionic bond formation between k (potassium,.
chemistry knowledge Comparison between Covalent and Ionic Bond
Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Ionic bonds form between nonmetals and metals. Web bonds between two nonmetals are generally covalent; The attraction of oppositely charged ions caused by electron. They form as a result of electrostatic attraction between oppositely charged ions and usually occur.
Ionic Bond Definition, Types, Properties & Examples
Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. So just choose the one with a nonmetal element (like na) and a metal element (like cl) yes, k and br. Web answered which of these pairs would form an ionic bond? Electron transfer produces.
Some Compounds Contain Both Covalent And Ionic Bonds.
These oppositely charged ions attract each other to form ionic networks (or lattices). Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web the tendency to form species that have eight electrons in the valence shell is called the octet rule. Web ionic bonds are one of the two main types of chemical bonds.
Web Bonds Between Two Nonmetals Are Generally Covalent;
Web br (bromine) belongs to viia, and has _____ 7 valence electrons, explain the process of ionic bond formation between k (potassium, a metal) and br (bromine, a nonmetal). So just choose the one with a nonmetal element (like na) and a metal element (like cl) yes, k and br. Predict whether each pair would form ionic. Web which of these pairs would form an ionic bond?
I Got It Right Duhhh Choose All The.
Web answered which of these pairs would form an ionic bond? Bonding between a metal and a nonmetal is often ionic. These oppositely charged ions attract each other to form ionic networks (or lattices. The attraction of oppositely charged ions caused by electron.
Ionic Bonds Form Between Nonmetals And Metals.
Web ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Electron transfer produces negative ions called anions and. K and br answers answer 1 is there any other pairs answer 2 answer:


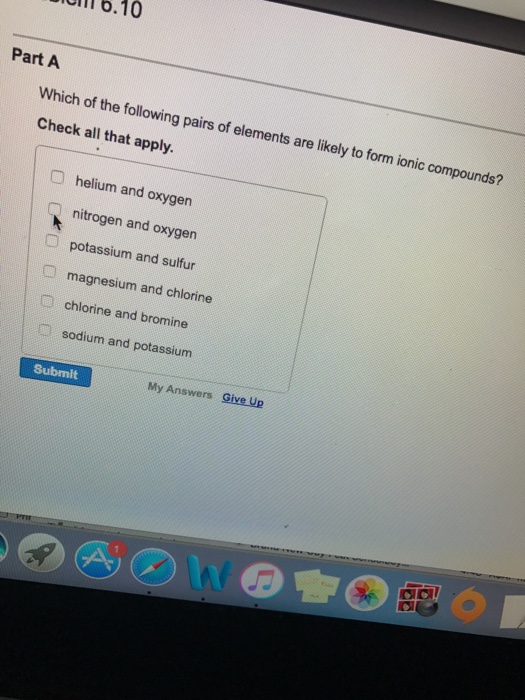



.PNG)