Why Do Metals Form Cations
Why Do Metals Form Cations - The solvation number, n, determined by a variety of. Energy is released when electrons are removed from metal ions. Web the alkali metals tend to form +1 cations. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. We will take a closer look at why is that so. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to react with simple lewis bases to form metal complexes. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. Web metallic atoms hold some of their electrons relatively loosely. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. This is actually one of the chemical properties.
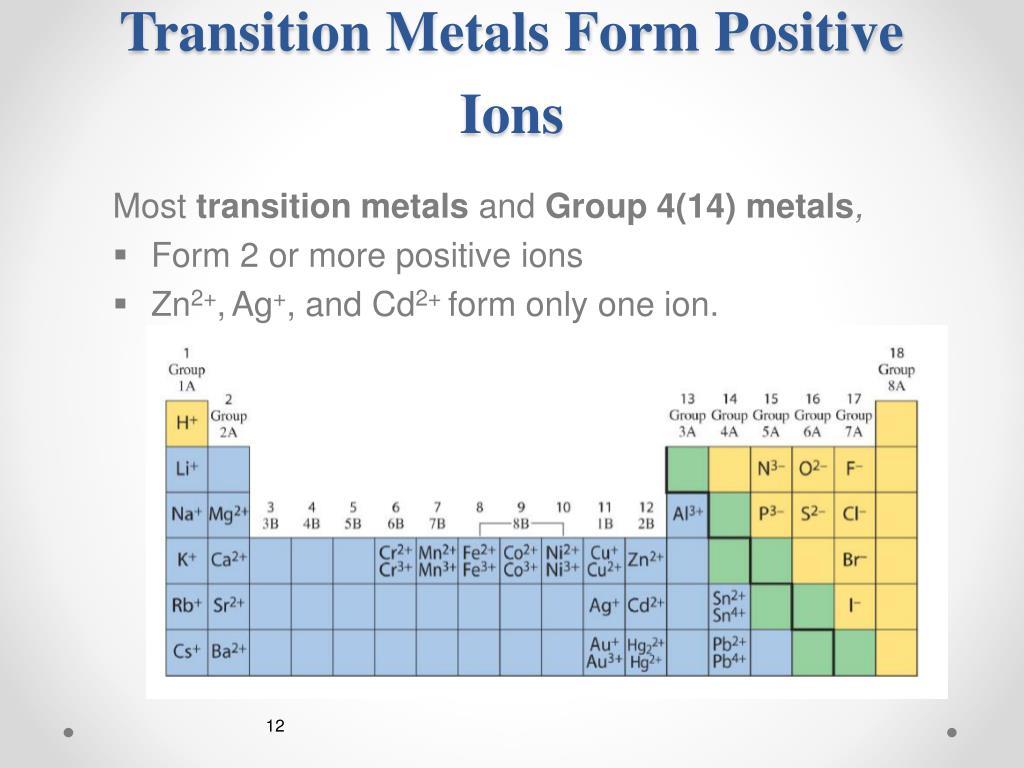
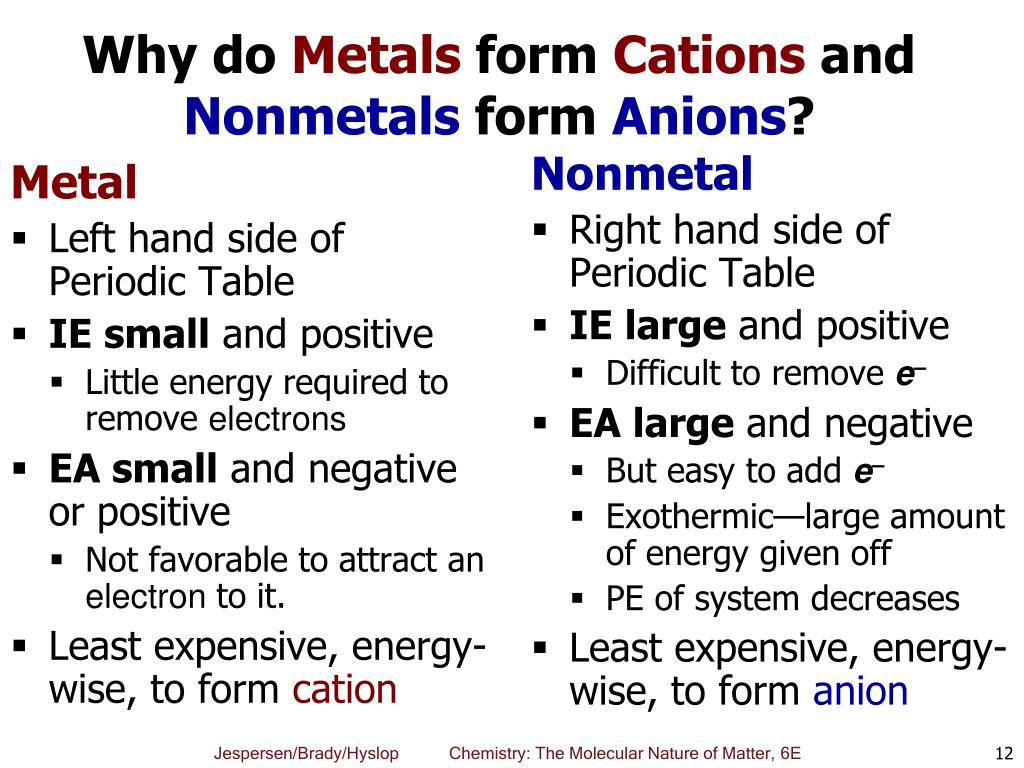
Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to react with simple lewis bases to form metal complexes. Web it is generally known that metals tend to form cations. Web alkali metals and alkaline earth metals always form cations. Potassium cations are common inside animal cells, and. Web acetylcholine is a cation that is a neurotransmitter, responsible for the transference of nerve impulses. These ions are positive because they contain more protons than electrons. Metals are good conductors because they have free electrons. Web metallic atoms hold some of their electrons relatively loosely. Silver and copper are the two best conductors of heat and electricity.
Potassium cations are common inside animal cells, and. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. This is the typical behavior. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. Most other nonmetals typically form anions (e.g. The more vigorous its reactions are; Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to react with simple lewis bases to form metal complexes. Consequently, they tend to lose electrons and form cations. Web the alkali metals tend to form +1 cations.
How To Find Electron Charge On Periodic Table Food Ideas
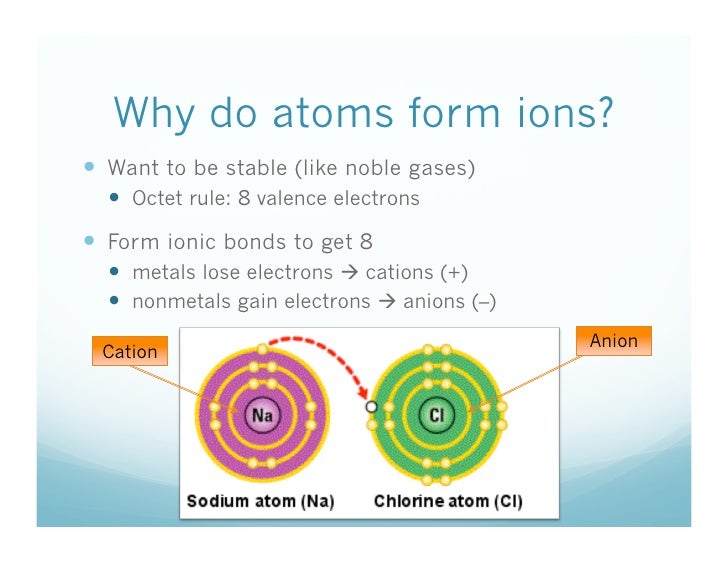
Web metallic atoms hold some of their electrons relatively loosely. Web atoms lose electrons from their outer shell when they form positive ions, called cations. This is the typical behavior. Potassium cations are common inside animal cells, and. Energy is released when electrons are removed from metal ions.
Solved Question 6 (1 point) Why do metals tend to form
Silver and copper are the two best conductors of heat and electricity. The more vigorous its reactions are; Web acetylcholine is a cation that is a neurotransmitter, responsible for the transference of nerve impulses. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. Web atoms lose electrons.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
Web alkali metals and alkaline earth metals always form cations. The solvation number, n, determined by a variety of. Metals are good conductors because they have free electrons. Most other nonmetals typically form anions (e.g. The more vigorous its reactions are;
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Web it is generally known that metals tend to form cations. Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Silver and copper are the two best conductors of heat and electricity. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have.
Do metals form anions or cations quizlet? Book Vea
Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Metals are good conductors because they have free electrons. This is the typical behavior. Web atoms lose electrons from their outer shell when they form positive ions, called cations. We will take a closer look at why is that.
Chem matters ch6_ionic_bond
It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Web atoms lose electrons from their outer shell when they form positive ions, called cations. Consequently, they tend to lose electrons and.
10 28 How Many Electrons Do Atoms Gain Lose
Web metallic atoms hold some of their electrons relatively loosely. The solvation number, n, determined by a variety of. Web it is generally known that metals tend to form cations. Metals are good conductors because they have free electrons. The more vigorous its reactions are;
is incorreer? all s area eleseis erand e are metals b, all p area
Web acetylcholine is a cation that is a neurotransmitter, responsible for the transference of nerve impulses. Energy is released when electrons are removed from metal ions. Web the alkali metals tend to form +1 cations. Web atoms lose electrons from their outer shell when they form positive ions, called cations. The more easily it loses electrons in reactions to form.
Why do metals form cations and non metals form anions?
Potassium cations are common inside animal cells, and. Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. The solvation number, n,.
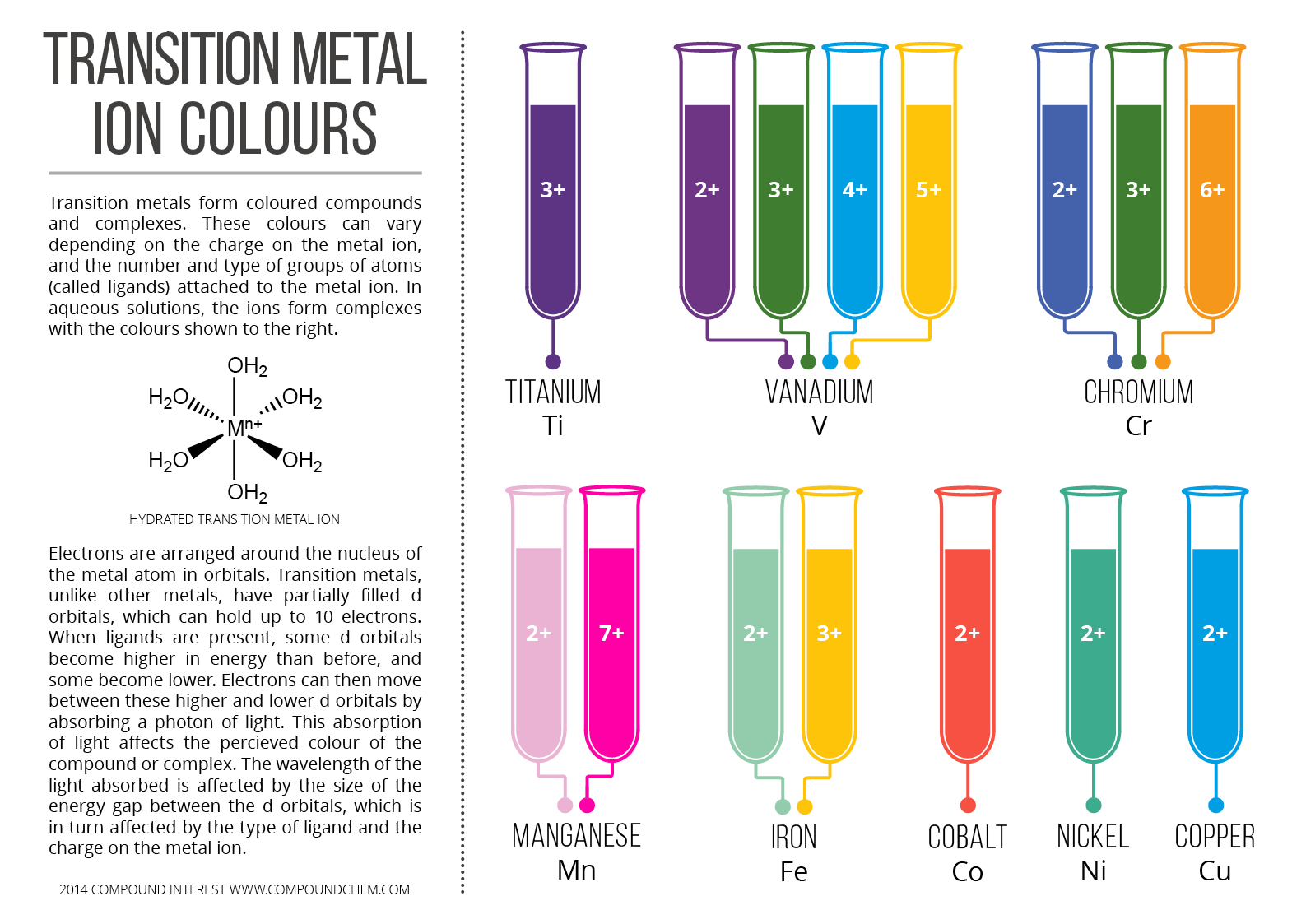
Colours of Transition Metal Ions in Aqueous Solution [Infographic
Silver and copper are the two best conductors of heat and electricity. This is the typical behavior. Metals are good conductors because they have free electrons. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. The more vigorous its reactions are;
We Will Take A Closer Look At Why Is That So.
Web metallic atoms hold some of their electrons relatively loosely. Metals are good conductors because they have free electrons. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Most other nonmetals typically form anions (e.g.
Consequently, They Tend To Lose Electrons And Form Cations.
This is the typical behavior. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. Web it is generally known that metals tend to form cations. Web the alkali metals tend to form +1 cations.
It Is Easiest To Achieve Noble Gas Configurations By Removing Valence Electrons From Metals (Which Have Few.
Silver and copper are the two best conductors of heat and electricity. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. These ions are positive because they contain more protons than electrons. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the.
Web Cations Are Atoms That Contain A Positive Charge, And They Are Formed When The Atoms Lose Electrons Which Are Negatively Charged.
Web acetylcholine is a cation that is a neurotransmitter, responsible for the transference of nerve impulses. Potassium cations are common inside animal cells, and. This is actually one of the chemical properties. The more vigorous its reactions are;