Xolair Enrollment Form 2022
Xolair Enrollment Form 2022 - Web xolair enrollment form date: Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. (a) patient has been established on therapy with xolair for nasal polyps under an active. Read “authorization to use and disclose personal information” on page 2. Web xolair® (omalizumab) enrollment form page 3 of 3 a division of health care service corporation, a mutual legal reserve company, an independent licensee of the blue. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Sign and date page 3. Web xolair will be approved based on one of the following criteria: This includes an open enrollment form and planned entry form. Easily fill out pdf blank, edit, and sign them.
Xolair is not indicated for treatment of other forms of urticaria. See full prescribing, safety, & boxed warning info. Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. This includes an open enrollment form and planned entry form. Web asthma enrollment form six simple steps to submitting a referral 1 (complete or include demographic sheet)patient information. Read “authorization to use and disclose personal information” on page 2. Web xolair will be approved based on one of the following criteria: Web xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: Web xolair enrollment form date: Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print).
Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic spontaneous urticaria who remain symptomatic despite h1 antihistamine. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Web xolair® (omalizumab) enrollment form page 3 of 3 a division of health care service corporation, a mutual legal reserve company, an independent licensee of the blue. Web asthma enrollment form six simple steps to submitting a referral 1 (complete or include demographic sheet)patient information. Web please follow these 3 steps to get started: Thu, 10 feb, 2022 at 8:05 am. Sign and date page 3. Web both the prescriber service form and the patient consent form must be received before xolair access solutions can begin helping your patient. Xolair is not indicated for treatment of other forms of urticaria. Please note you must sign the.
Fillable Xolair Request Form Blue Cross & Blue Shield printable pdf
Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. Once completed, fax to the number indicated on the form. This includes an open enrollment form and planned entry form. Thu, 10 feb, 2022 at 8:05 am. Read “authorization to use and disclose personal information” on page 2.
Xolair Update asthmablog1971
Easily fill out pdf blank, edit, and sign them. Thu, 10 feb, 2022 at 8:05 am. See full prescribing, safety, & boxed warning info. Web sign up to receive patient support resources, including information on getting started with xolair® (omalizumab). Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic spontaneous.
Xolair Patient Consent Form 2023
Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic spontaneous urticaria who remain symptomatic despite h1 antihistamine. Web sign up to receive patient support resources, including information on getting started with xolair® (omalizumab). Once completed, fax to the number indicated on the form. Please note you must sign the. Web.
Xolair Enrollment Form Enrollment Form
Easily fill out pdf blank, edit, and sign them. (a) patient has been established on therapy with xolair for nasal polyps under an active. Read “authorization to use and disclose personal information” on page 2. Thu, 10 feb, 2022 at 8:05 am. Save or instantly send your ready documents.
XOLAIR (omalizumab) XHALE PSP Form 2022 World OSCAR
(a) patient has been established on therapy with xolair for nasal polyps under an active. Save or instantly send your ready documents. (1) all of the following: Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Read “authorization to use and disclose personal information” on.
Enrollment for 1st Semester A.Y. 20212022 is now OPEN. BAGUIO
Web xolair will be approved based on one of the following criteria: The bias introduced by allowing enrollment of patients previously exposed to xolair. Easily fill out pdf blank, edit, and sign them. Read “authorization to use and disclose personal information” on page 2. Web xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat:
Enrollment Form For Xolair Enrollment Form
Web both the prescriber service form and the patient consent form must be received before xolair access solutions can begin helping your patient. Twelvestone health partners fax referral to: Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. Web xolair will be approved based on one of the following criteria: See full.
XOLAIR Dosage & Rx Info Uses, Side Effects The Clinical Advisor
(1) all of the following: Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. The bias introduced by allowing enrollment of patients previously exposed to xolair. Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic spontaneous urticaria who remain symptomatic despite h1.
Xolair Injection Latest Price, Dealers & Retailers in India
Save or instantly send your ready documents. Once completed, fax to the number indicated on the form. See full prescribing, safety, & boxed warning info. Please note you must sign the. Web xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat:
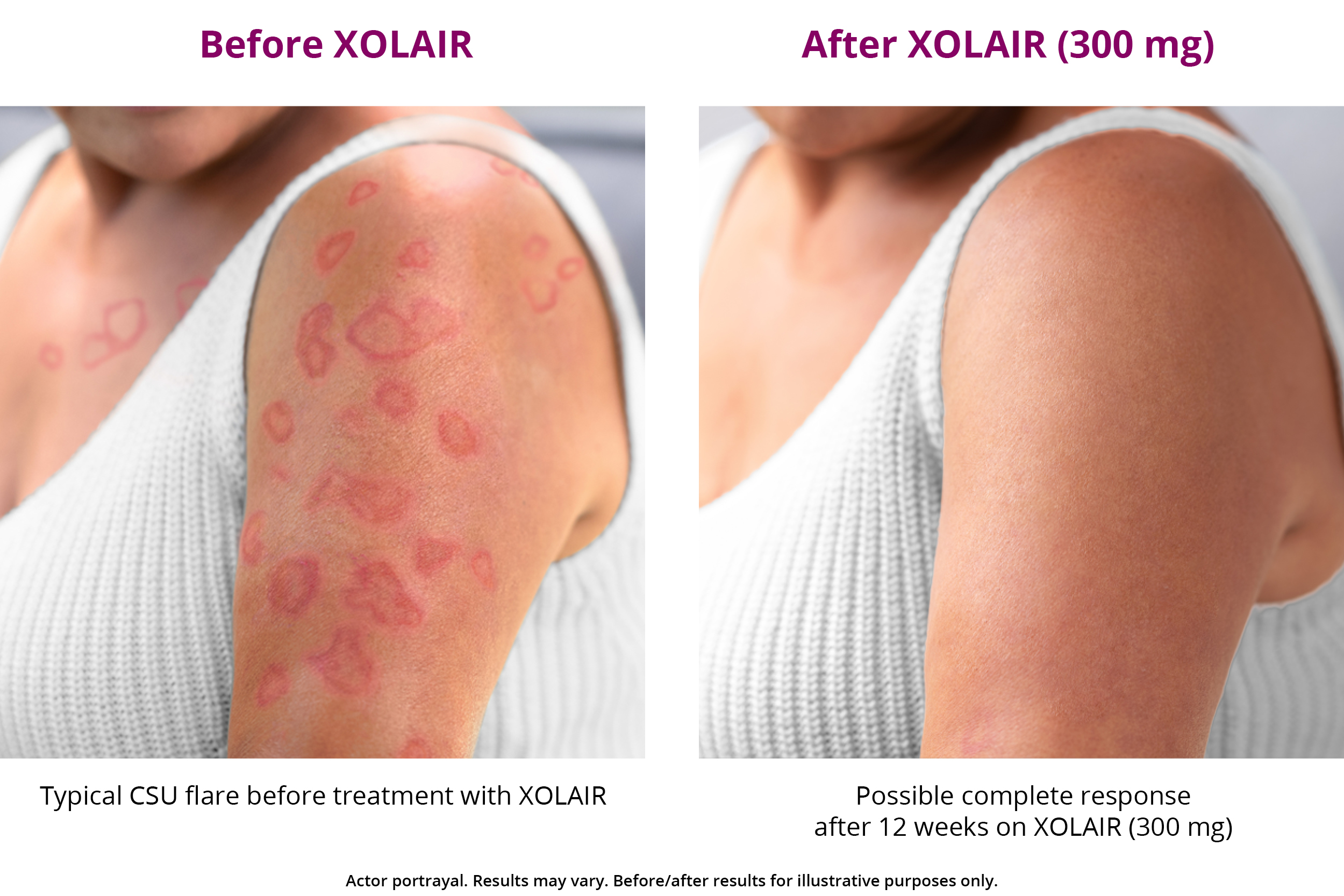
XOLAIR CSU Treatment Results XOLAIR® (omalizumab)
Please print and complete the forms below. Twelvestone health partners fax referral to: (a) patient has been established on therapy with xolair for nasal polyps under an active. Read “authorization to use and disclose personal information” on page 2. Once completed, fax to the number indicated on the form.
Thu, 10 Feb, 2022 At 8:05 Am.
Read “authorization to use and disclose personal information” on page 2. Web xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. Twelvestone health partners fax referral to:
Easily Fill Out Pdf Blank, Edit, And Sign Them.
Web both the prescriber service form and the patient consent form must be received before xolair access solutions can begin helping your patient. (1) all of the following: Web ☐ this signed order form ☐ history and physical ☐ patient demographics and insurance information ☐ clinicalprogress notes, lab work (including most recent renal function tests. Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic spontaneous urticaria who remain symptomatic despite h1 antihistamine.
Xolair Is Not Indicated For Treatment Of Other Forms Of Urticaria.
The bias introduced by allowing enrollment of patients previously exposed to xolair. Web asthma enrollment form six simple steps to submitting a referral 1 (complete or include demographic sheet)patient information. Please print and complete the forms below. Web sign up to receive patient support resources, including information on getting started with xolair® (omalizumab).
Once Completed, Fax To The Number Indicated On The Form.
Web xolair enrollment form date: See full prescribing, safety, & boxed warning info. Sign and date page 3. (a) patient has been established on therapy with xolair for nasal polyps under an active.