Xolair Patient Enrollment Form
Xolair Patient Enrollment Form - Xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: Web xolair will be approved based on the following criterion: • adult and pediatric patients (6 years of age and above) with moderate to severe persistent asthma. Web this service offers coverage support, patient assistance, and other useful information. Review the dosing schedule and your administration options. Blue cross and blue shield of texas. Please print and complete the forms below. See full prescribing, safety, & boxed warning info. Web with my patient solutions, you can: Web download the forbearing consent form to begin enrollment with xolair access solutions.
Please print and complete the forms below. Web sign up to receive patient support resources, including information on getting started with xolair® (omalizumab). Web patient enrollment forms | xolair access solutions forms and documents download the form you need to enroll in genentech access solutions. Web xolair will be approved based on the following criterion: Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). • adult and pediatric patients (6 years of age and above) with moderate to severe persistent asthma. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). For patients prescribed prxolair® for moderate to severe allergic asthma (aa) or chronic idiopathic urticaria. The bias introduced by allowing enrollment of patients previously exposed to. Web download the forbearing consent form to begin enrollment with xolair access solutions.
Xolair® (omalizumab) fax completed form to 866.531.1025. Web xhale+ program patient enrolment and consent form: For patients prescribed prxolair® for moderate to severe allergic asthma (aa) or chronic idiopathic urticaria. Web xolair® (omalizumab) enrollment form xolair® (omalizumab) enrollment form fax completed form to: Web download of patient consent form to begin enrollment with xolair admittance choose. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. View benefits investigation (bi) reports; Ad visit the patient site to learn how the fasenra pen works. Xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: Review the dosing schedule and your administration options.
Xolair Patient Consent Form 2023
Web xhale+ program patient enrolment and consent form: Ad visit the patient site to learn how the fasenra pen works. Xolair® (omalizumab) fax completed form to 866.531.1025. See full prescribing, safety, & boxed warning info. Review the dosing schedule and your administration options.
XOLAIR Dosage & Rx Info Uses, Side Effects MPR
Ad proudly helping members navigate prescription assistance programs for 15 years! Blue cross and blue shield of texas. Genentech patient foundation provides free medicine to patients without. • adult and pediatric patients (6 years of age and above) with moderate to severe persistent asthma. Moderate to severe persistent asthma in people 6.
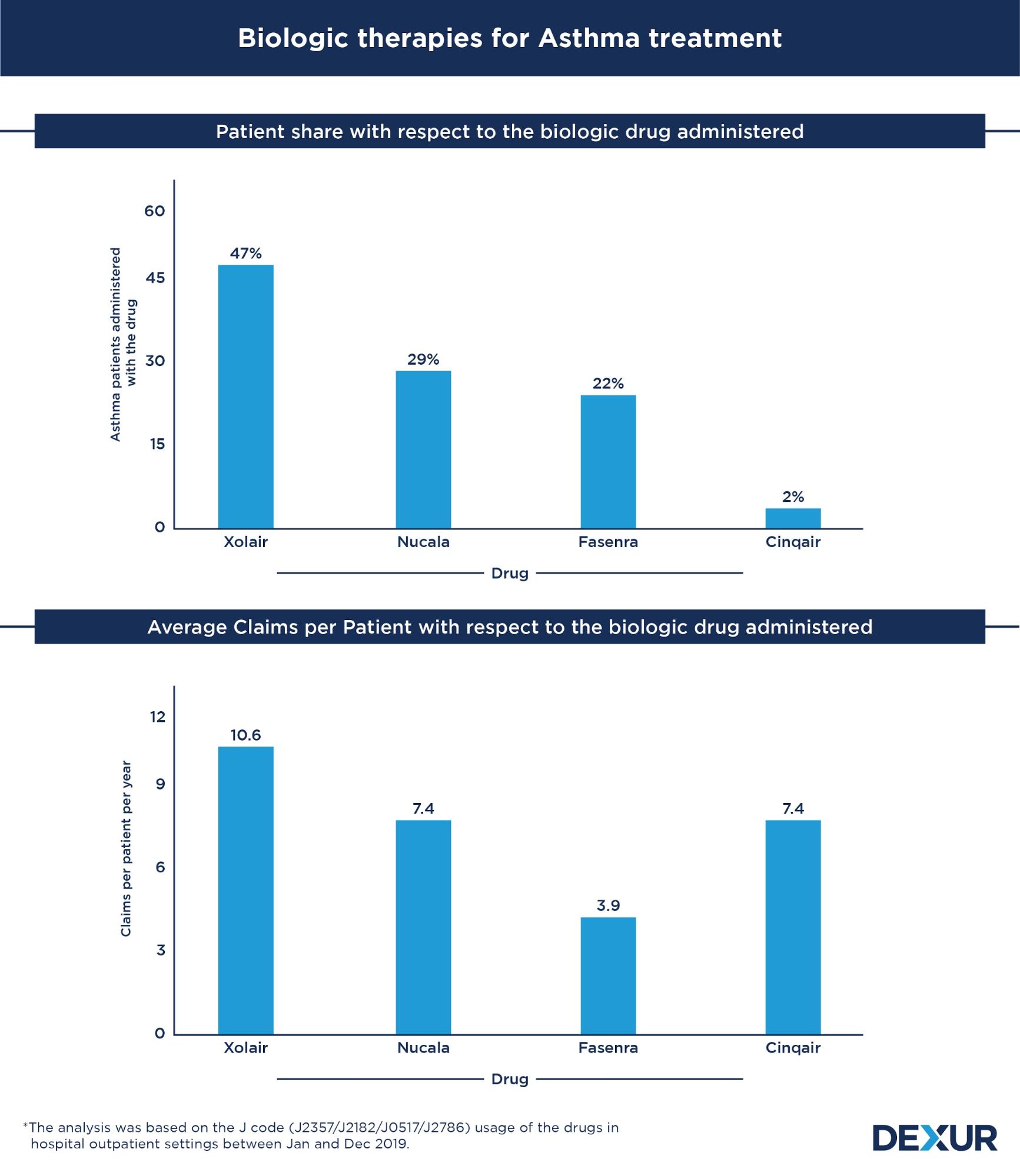
Xolair patient share was 1.6x more than Nucala and 2x compared to
Your patient’s benefit plan requires prior authorization for certain medications. Genentech patient foundation provides free medicine to patients without. Ad proudly helping members navigate prescription assistance programs for 15 years! Xolair® (omalizumab) fax completed form to 866.531.1025. Web xhale+ program patient enrolment and consent form:
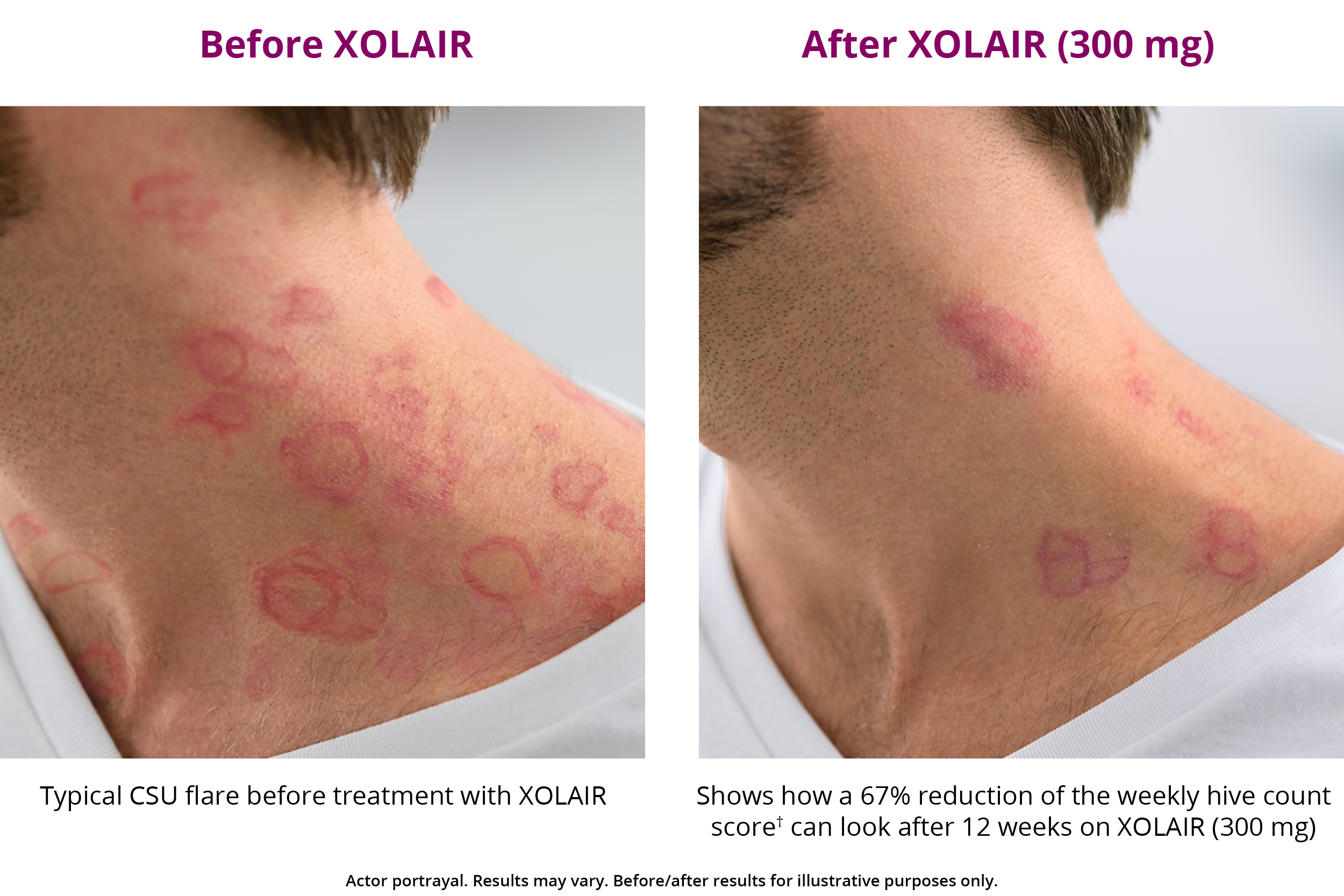
XOLAIR CSU Treatment Results XOLAIR® (omalizumab)
Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Web 1 of 2 prescription & enrollment form: Your patient’s benefit plan requires prior authorization for certain medications. Web with my patient solutions, you can: Web xolair® (omalizumab) enrollment form xolair® (omalizumab) enrollment form fax completed.
Xhale+ Xolair Enrolment Consent Form Cloud Practice
Once completed, fax to the number indicated on the form. Blue cross and blue shield of texas. Ad proudly helping members navigate prescription assistance programs for 15 years! Review the dosing schedule and your administration options. View benefits investigation (bi) reports;
Chronic Spontaneous Urticaria Treatment XOLAIR® (omalizumab)
Genentech patient foundation provides free medicine to patients without. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Once completed, fax to the number indicated on the form. Blue cross and blue shield of texas. For patients prescribed prxolair® for moderate to severe allergic asthma (aa) or chronic.
Enrollment Form For Xolair Enrollment Form
Once completed, fax to the number indicated on the form. Xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: Web xolair® (omalizumab) enrollment form xolair® (omalizumab) enrollment form fax completed form to: View benefits investigation (bi) reports; Blue cross and blue shield of texas.
Xolair Dose Table Wallseat.co
See full prescribing, safety, & boxed warning info. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). In order to make appropriate medical necessity determinations,. Web download of patient consent form to begin enrollment with xolair admittance choose. Moderate to severe persistent asthma in people.
Xolair Enrollment Form Enrollment Form
Web patient enrollment forms | xolair access solutions forms and documents download the form you need to enroll in genentech access solutions. Patient’s first name last name middle initial date of birth prescriber’s first. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Web the first step is.
Why Every Xolair Patient Should Keep an Allergy Journal IVX Health
See full prescribing, safety, & boxed warning info. Web the first step is to have patients complete and submit the respiratory patient consent form. The bias introduced by allowing enrollment of patients previously exposed to. Ad visit the patient site to learn how the fasenra pen works. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in.
Committed To Helping Patients Access The Xolair They Have Been Prescribed.
Web 1 of 2 prescription & enrollment form: Xolair® (omalizumab) fax completed form to 866.531.1025. In order to make appropriate medical necessity determinations,. Once completed, fax to the number indicated on the form.
Web This Service Offers Coverage Support, Patient Assistance, And Other Useful Information.
Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Patient’s first name last name middle initial date of birth prescriber’s first. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Web the first step is to have patients complete and submit the respiratory patient consent form.
Web Sign Up To Receive Patient Support Resources, Including Information On Getting Started With Xolair® (Omalizumab).
Ad visit the patient site to learn how the fasenra pen works. The bias introduced by allowing enrollment of patients previously exposed to. Web download of patient consent form to begin enrollment with xolair admittance choose. Web xolair will be approved based on the following criterion:
Moderate To Severe Persistent Asthma In People 6.
Web download the forbearing consent form to begin enrollment with xolair access solutions. View and track your patient cases; Genentech patient foundation provides free medicine to patients without. Xolair ® (omalizumab) for subcutaneous use is an injectable prescription medicine used to treat: