Zwitterion Form Of Amino Acids
Zwitterion Form Of Amino Acids - Athletics are very competitive these days at all levels, from school sports to the pros. Web the existence of amino acids as dipolar ions in a solid state is called zwitterions. Web zwitterion amino acids. The structure of an amino acid allows it to act as both an acid and a base. Web stabilization of zwitterionic structures of a series of amino acids (gly, ala, val, ser, and pro) by various ammonium ions (nh 4 +, ch 3 nh 3 +, (ch 3) 2 nh 2 +, and (ch 3) 3 nh +) has been investigated systematically. Apart from amino acids, any compound that contains acid and base centres can obtain a zwitterion form. Rch(nh 2)co 2 h ⇌ rcn(n + h 3)co − 2. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna) translation (scot et al., 2006). Web draw the zwitterion form of a given amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation.
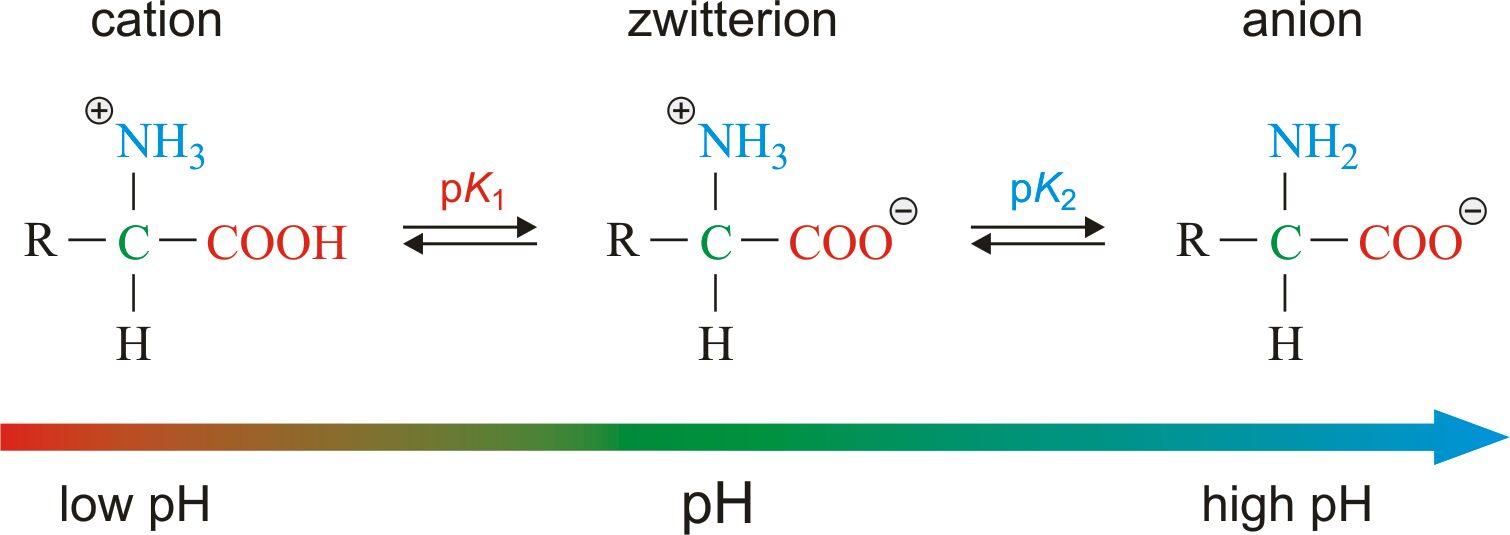
An amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. They contain an amine group (basic) and a carboxylic group (acidic). Web video quiz course 27k views amino acids as zwitterions amino acids are the building blocks of proteins in living cells. Web to explain how an amino acid can act as both an acid and a base. Common zwitterions are amino acids. Athletics are very competitive these days at all levels, from school sports to the pros. The ratio of the concentrations of the two species in solution is independent of ph. Web amino acids an amino acid contains both acidic (carboxylic acid fragment) and basic (amine fragment) centres. This is the form that amino acids exist in even in the solid state. Figures 3 and 4 show the distribution of species graph for clioquinol (an ampholyte) and ampicillin (a zwitterion).
The accepted practice is to show the amino acids in the zwitterion form. Web an amino acid has both a basic amine group and an acidic carboxylic acid group. Some more examples include tricine, bicine, solid sulfamic acid, alkaloids like psilocybin amongst others. This is the form that amino acids exist in even in the solid state. Web zwitterion form of amino acids andrey k 710k subscribers subscribe 376 share 35k views 8 years ago donate here: Tautomerism of amino acids follows this stoichiometry: Athletics are very competitive these days at all levels, from school sports to the pros. Web video quiz course 27k views amino acids as zwitterions amino acids are the building blocks of proteins in living cells. Web zwitterion amino acids. Web it results due to the neutralization reaction in amino acid.
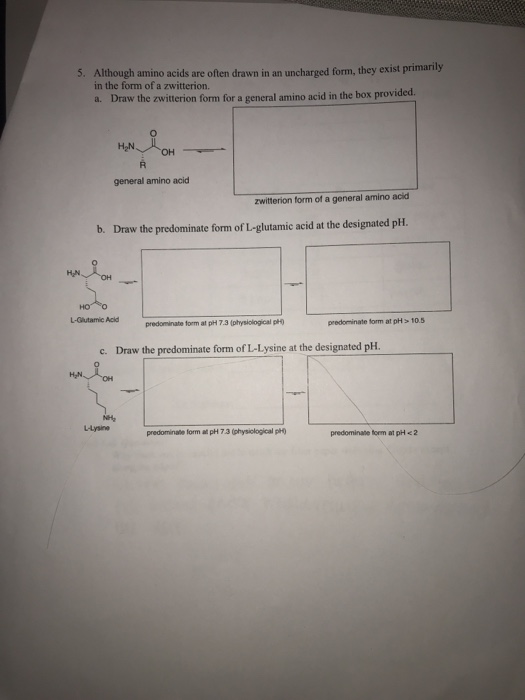
Solved Although amino acids are often drawn in an uncharged
(1) the carboxyl group can lose a hydrogen ion to become negatively charged. Figures 3 and 4 show the distribution of species graph for clioquinol (an ampholyte) and ampicillin (a zwitterion). Web video quiz course 27k views amino acids as zwitterions amino acids are the building blocks of proteins in living cells. Web the zwitterion form of an amino acid.
Pin on MCAT Bio / Biochemistry Tutorials and Resources
An amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. The accepted practice is to show the amino acids in the zwitterion form. Web basic amino acids have isoionic points larger than 7. Both molecules have two pka values. Likewise, we can have tripeptides,.
Zwitterion Definition and Examples
The structure of an amino acid allows it to act as both an acid and a base. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. Web a zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged. Web.
Zwitterion Form of Amino Acids YouTube
Web a zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. For basic amino acids, the basic functional group in the side chain tends to be protonated at a.
Amino Acid In Zwitterion Form Integrated MCAT Course
Both molecules have two pka values. This is the form that amino acids exist in even in the solid state. The ratio of the concentrations of the two species in solution is independent of ph. The isomer on the right is a zwitterion. Web a zwitterion is a molecule with functional groups, of which at least one has a positive.
Proteins Broad Learnings
Likewise, we can have tripeptides, tetrapeptides. For basic amino acids, the basic functional group in the side chain tends to be protonated at a ph near neutrality. The ratio of the concentrations of the two species in solution is independent of ph. This is the form that amino acids exist in even in the solid state. The structure of an.
Zwitterionic Structures of Amino Acids. Download Scientific Diagram
This is the form that amino acids exist in even in the solid state. Athletics are very competitive these days at all levels, from school sports to the pros. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The ratio of the concentrations of the two species.
18.1 Properties of Amino Acids The Basics of General, Organic, and
They are compounds that contain an amino group and a carboxyl group. Common zwitterions are amino acids. Web ampholytes and zwitterions are molecules with at least two pka values, at least one of which is acidic and at least one is basic. Determine the charge on an amino acid when it is not at the isoelectric point. To evaluate whether.
zwitterion YouTube
Web an amino acid has both a basic amine group and an acidic carboxylic acid group. Thus, there are two ammonium ions and only one carboxylate ion. When two amino acids link together to form an amide link, the resulting structure is called a dipeptide. Likewise, we can have tripeptides, tetrapeptides. Athletics are very competitive these days at all levels,.
Amino acids physical, chemical properties and peptide bond
When two amino acids link together to form an amide link, the resulting structure is called a dipeptide. It's not that the oxygen 'wants' to lose a proton, but more that at that $\mathrm{ph}$ the equilibrium lies towards the deprotonated state (things are rarely. This is called a zwitterion. You will learn how to calculate the isoelectric point, and the.
Web The Existence Of Amino Acids As Dipolar Ions In A Solid State Is Called Zwitterions.
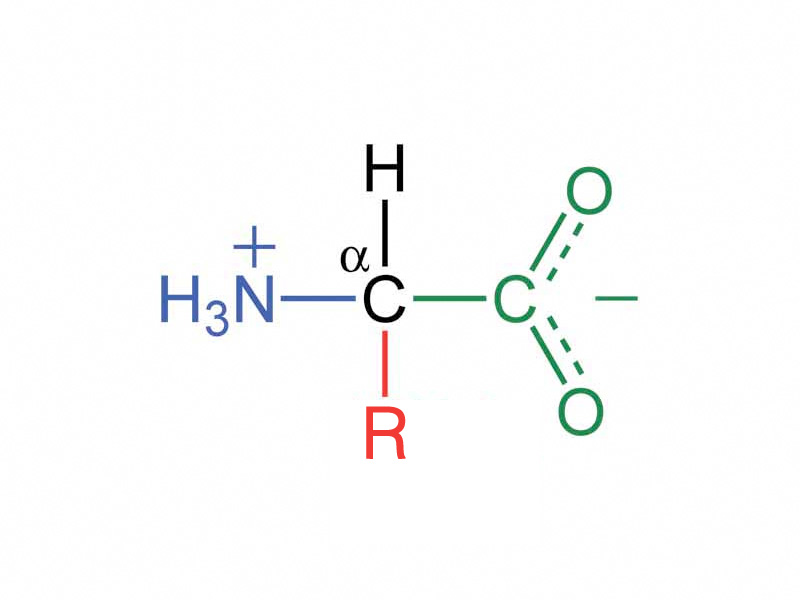
The basic structure of an amino acid includes a carbon attached to an amino group, a carboxylic acid group, an r group, and hydrogen: Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Web extensive studies on amino acids indicate that, in solution, the zwitterion is the predominant species of the neutral form of the amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation.
Common Zwitterions Are Amino Acids.
Web zwitterion form of amino acids andrey k 710k subscribers subscribe 376 share 35k views 8 years ago donate here: At different ph, the structures of amino acids change because of ionisable nature of −nh2 and −cooh groups. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna) translation (scot et al., 2006). Web a zwitterion is a molecule with functional groups, of which at least one has a positive and one has a negative electrical charge.
Web Video Quiz Course 27K Views Amino Acids As Zwitterions Amino Acids Are The Building Blocks Of Proteins In Living Cells.
Web it results due to the neutralization reaction in amino acid. An amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. Athletics are very competitive these days at all levels, from school sports to the pros. Web a zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged.
The Accepted Practice Is To Show The Amino Acids In The Zwitterion Form.
They contain an amine group (basic) and a carboxylic group (acidic). Some more examples include tricine, bicine, solid sulfamic acid, alkaloids like psilocybin amongst others. Rch(nh 2)co 2 h ⇌ rcn(n + h 3)co − 2. Web an amino acid has both a basic amine group and an acidic carboxylic acid group.