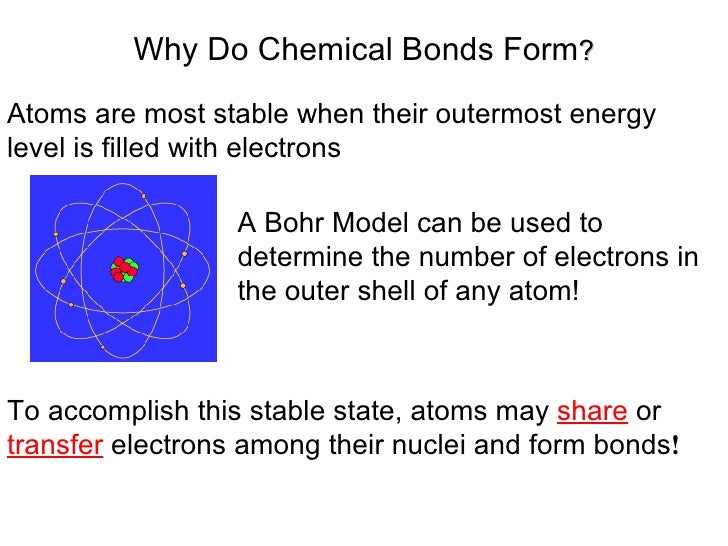
Why Do Atoms Form Bonds
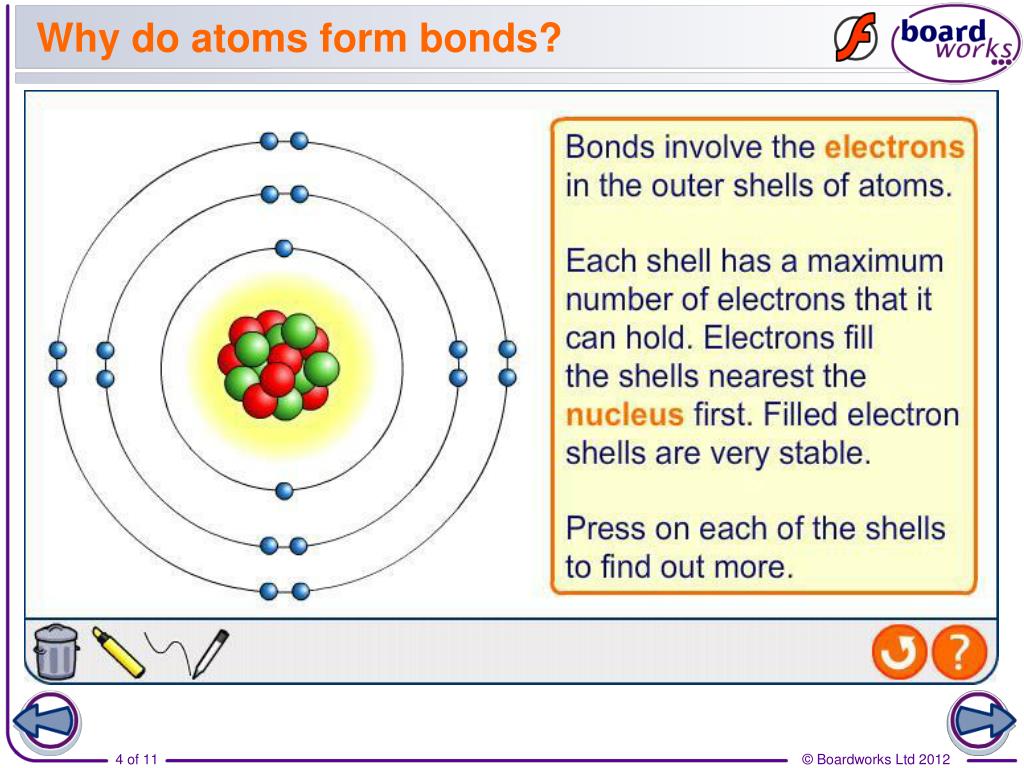
Why Do Atoms Form Bonds - Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy. They want a full outer shell of electrons, so they lose, gain, or share electrons with other elements, forming compounds, until they have 8 valence electrons and become stable. Web why do atoms form chemical bonds? Web there are three basic ways that the outer electrons of atoms can form bonds: Continue learning with ulearngo [attributions and licenses] chemical bonds continued An ionic bond, where one atom essentially donates an electron to another, forms when one atom becomes stable by losing its outer electrons and the other atoms become stable (usually by filling. Web why form chemical bonds? A second reason atoms form bonds is that they are able to donate electrons to, or receive electrons from, other atoms to complete their respective valence shells. Atoms with a full valence electron orbital are less reactive.
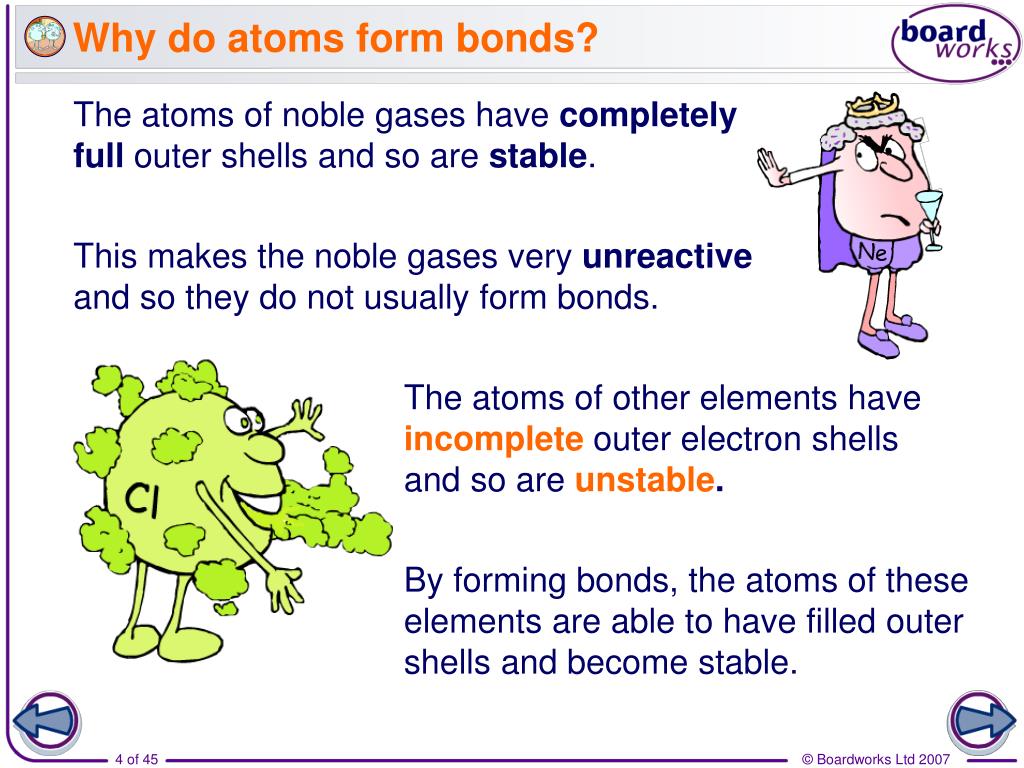
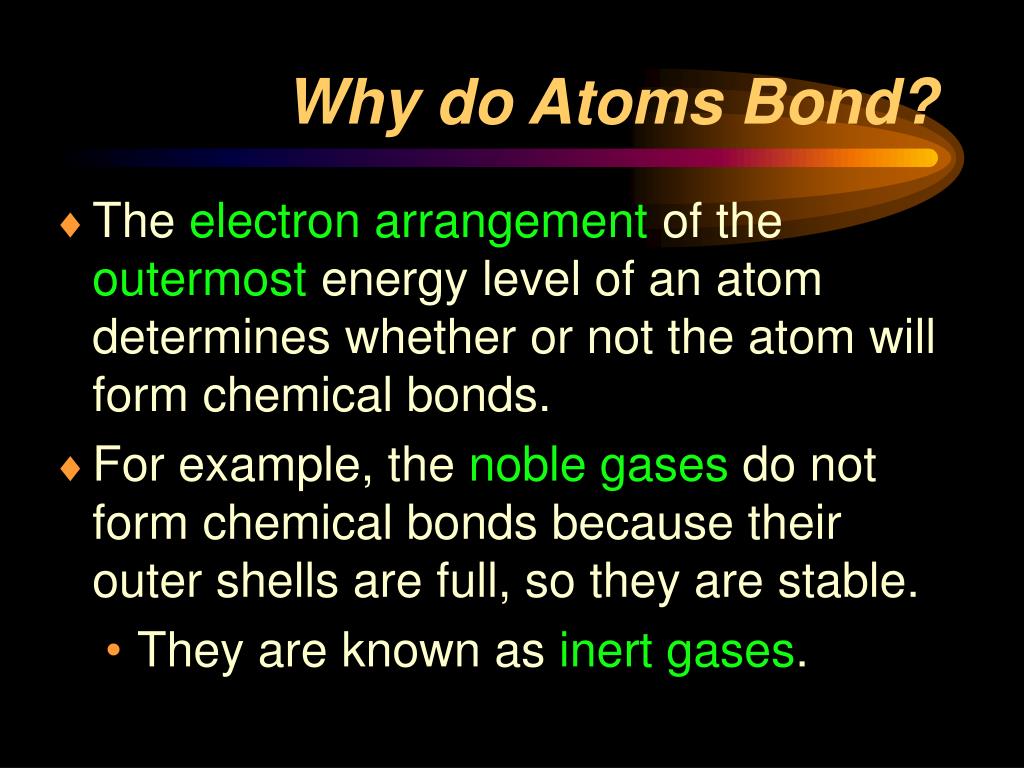
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web when atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative arrangement. Got questions about this content? These bonds are usually stronger than covalent bonds because of the electronegativity difference between them (the physical impetus for a donation rather. Web why do atoms form chemical bonds? Web why do atoms form chemical bonds? A second reason atoms form bonds is that they are able to donate electrons to, or receive electrons from, other atoms to complete their respective valence shells. Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. Atoms with a full valence electron orbital are less reactive. Web atoms form bonds to try to achieve the same electron configuration as the noble gases.
Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. Web atoms form bonds to try to achieve the same electron configuration as the noble gases. Got questions about this content? Web why form chemical bonds? An ionic bond, where one atom essentially donates an electron to another, forms when one atom becomes stable by losing its outer electrons and the other atoms become stable (usually by filling. That chemical bonding involves atoms being held together by electrostatic forces (positive charges attracting negative charges), which become stronger as the magnitudes of these charges become stronger. Web why do atoms form chemical bonds? Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web there are three basic ways that the outer electrons of atoms can form bonds: The first way gives rise to what is called an ionic bond.
PPT Ions PowerPoint Presentation, free download ID6738771
Web bonds form when atoms share or transfer valence electrons. Web when atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative arrangement. Atoms with a full valence electron orbital are less reactive. Web that atoms must.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy. They want a full outer shell of electrons, so they lose, gain, or share electrons with other elements, forming compounds, until they have 8 valence electrons and become stable. The first way.
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609
They want a full outer shell of electrons, so they lose, gain, or share electrons with other elements, forming compounds, until they have 8 valence electrons and become stable. Web the ionic bond: Web atoms form bonds to try to achieve the same electron configuration as the noble gases. Web why do atoms form chemical bonds? The type of chemical.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Web atoms form chemical bonds to make their outer electron shells more stable. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. 149) why does the octet rule not always refer to a stable arrangement of eight valence electrons? Web bonds form when atoms share or transfer valence electrons. There.
PPT Chemical Bonding What and Why PowerPoint Presentation, free
Web that atoms must collide (bump together) in order to form chemical bonds. Web why do atoms form chemical bonds? They want a full outer shell of electrons, so they lose, gain, or share electrons with other elements, forming compounds, until they have 8 valence electrons and become stable. There are three different types of chemical bonds:. Web the ionic.
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Atoms with a full valence electron orbital are less reactive. There are three different types of chemical bonds:. The type of chemical bond maximizes the stability of the atoms that form it. They want a full outer shell of electrons, so.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Web why do atoms form chemical bonds? Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web why do atoms form chemical bonds? Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. These bonds.
Biochemistry Honors
A second reason atoms form bonds is that they are able to donate electrons to, or receive electrons from, other atoms to complete their respective valence shells. Web that atoms must collide (bump together) in order to form chemical bonds. They want a full outer shell of electrons, so they lose, gain, or share electrons with other elements, forming compounds,.
Why Do Atoms Bond? YouTube
If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Atoms with a full valence electron orbital are less reactive. Many atoms become.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy. Web why form chemical bonds? Web when atoms approach one another, their nuclei.
Atoms Form Chemical Bonds To Achieve A Full Outer Energy Level, Which Is The Most Stable Arrangement Of Electrons.
A second reason atoms form bonds is that they are able to donate electrons to, or receive electrons from, other atoms to complete their respective valence shells. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. The type of chemical bond maximizes the stability of the atoms that form it. That chemical bonding involves atoms being held together by electrostatic forces (positive charges attracting negative charges), which become stronger as the magnitudes of these charges become stronger.
Web Bonds Form When Atoms Share Or Transfer Valence Electrons.
Web there are three basic ways that the outer electrons of atoms can form bonds: Web why do atoms form chemical bonds? Atoms with a full valence electron orbital are less reactive. An ionic bond, where one atom essentially donates an electron to another, forms when one atom becomes stable by losing its outer electrons and the other atoms become stable (usually by filling.
149) Why Does The Octet Rule Not Always Refer To A Stable Arrangement Of Eight Valence Electrons?
Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. Web atoms form chemical bonds to make their outer electron shells more stable. Web why do atoms form chemical bonds? Web that atoms must collide (bump together) in order to form chemical bonds.
They Want A Full Outer Shell Of Electrons, So They Lose, Gain, Or Share Electrons With Other Elements, Forming Compounds, Until They Have 8 Valence Electrons And Become Stable.
Web atoms form bonds to try to achieve the same electron configuration as the noble gases. These bonds are usually stronger than covalent bonds because of the electronegativity difference between them (the physical impetus for a donation rather. Atoms form chemical bonds because they want a lower energy and more stable electron configuration. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy.